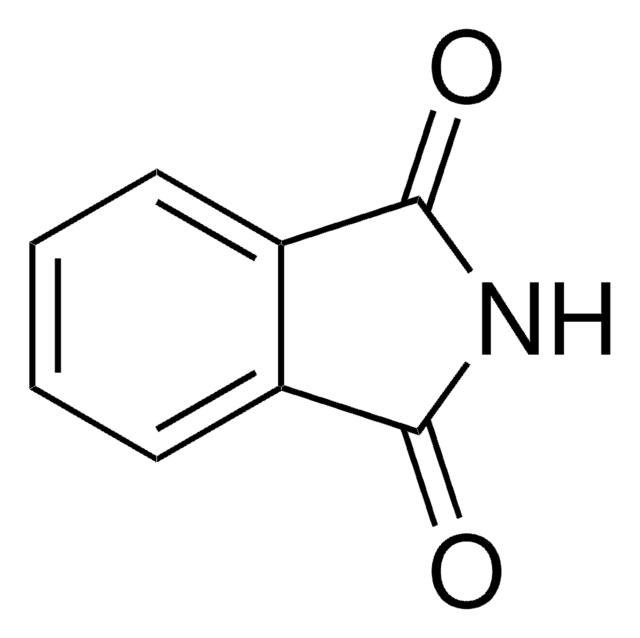
252611
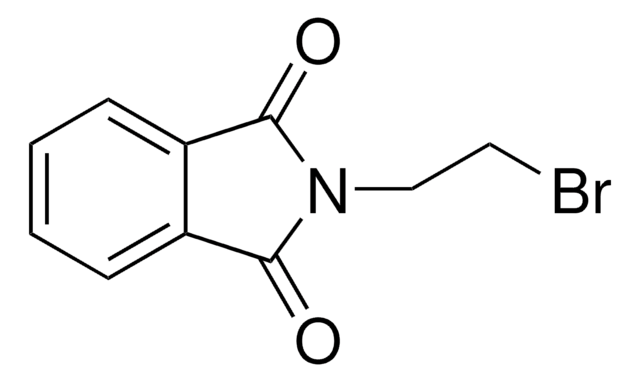
N-(Bromomethyl)phthalimide
96%
Synonym(s):
Phthalimidomethyl bromide
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C9H6BrNO2
CAS Number:
Molecular Weight:
240.05
Beilstein:
140943
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
96%
form
solid
mp
152-155 °C (lit.)
SMILES string
BrCN1C(=O)c2ccccc2C1=O
InChI
1S/C9H6BrNO2/c10-5-11-8(12)6-3-1-2-4-7(6)9(11)13/h1-4H,5H2
InChI key
UUSLLECLCKTJQF-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
N-(Bromomethyl)phthalimide was used as initiator in synthesis of α-phthalimidopoly(styrene) by atom transfer radical polymerisation. It was also used in synthesis of functionalized 5-(aminomethyl)pyrimidine-2,4,6-trione analog and new bis-C(cage)-substituted o-carborane.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Synthesis and crystal structure of 1, 2-bis (phthalimidomethyl)-1, 2-dicarba-closo-dodecaborane (12): a precursor to polyamines.
Zhu Y, et al.
Inorganic Chemistry Communications, 4(8), 447-449 (2001)
James J-W Duan et al.
Bioorganic & medicinal chemistry letters, 17(1), 266-271 (2006-10-10)
Using a pyrimidine-2,4,6-trione motif as a zinc-binding group, a series of selective inhibitors of tumor necrosis factor-alpha converting enzyme (TACE) was discovered. Optimization of initial lead 1 resulted in a potent inhibitor (51), with an IC(50) of 2 nM in
Approaches to phthalimido and amino end-functional polystyrene by atom transfer radical polymerisation (ATRP).
Postma A, et al.
Reactive functional Polymers, 66(1), 137-147 (2006)
Leonardo P Giglio et al.
Nitric oxide : biology and chemistry, 98, 41-49 (2020-03-10)
Polymeric biomaterials capable of delivering nitric oxide (NO) topically can be used to enhance skin blood flow (SkBF) and accelerate wound healing. Herein, we used reversible addition-fragmentation chain transfer radical (RAFT) polymerization to synthesize the first poly(vinyl alcohol) (PVA) functionalized
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service