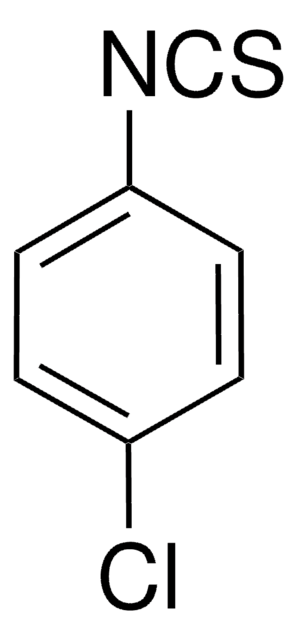
252492
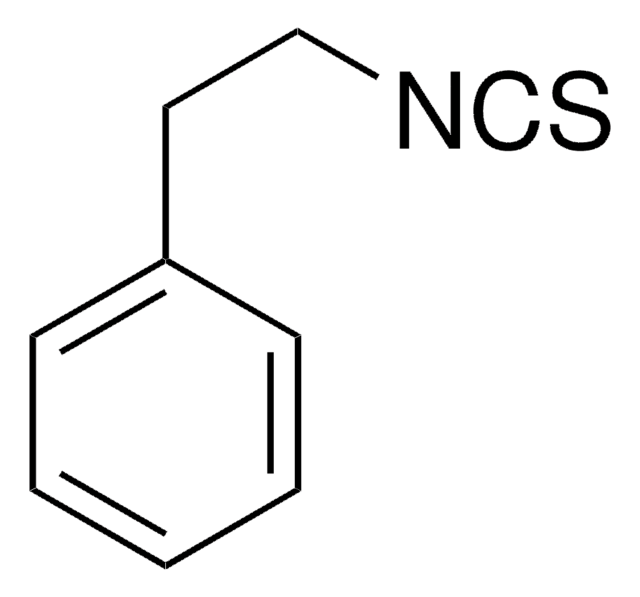
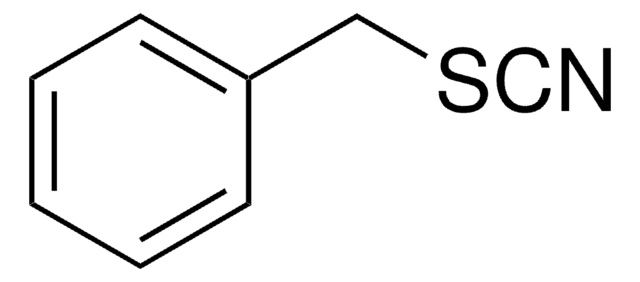
Benzyl isothiocyanate
98%
Synonym(s):
Benzyl mustard oil, Isothiocyanotaomethylbenzene
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
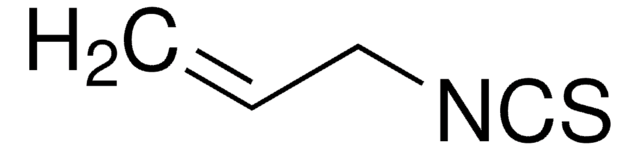
C6H5CH2NCS
CAS Number:
Molecular Weight:
149.21
Beilstein:
386135
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
form
liquid
refractive index
n20/D 1.601 (lit.)
bp
242-243 °C (lit.)
density
1.125 g/mL at 25 °C (lit.)
storage temp.
2-8°C
SMILES string
S=C=NCc1ccccc1
InChI
1S/C8H7NS/c10-7-9-6-8-4-2-1-3-5-8/h1-5H,6H2
InChI key
MDKCFLQDBWCQCV-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Benzyl isothiocyanate is a naturally-occurring constituent of cruciferous vegetables. It has antibacterial properties and its metabolism in man has been investigated. It inhibits chemically induced cancer in animal models.
Application
Benzyl isothiocyanate can be used as a reactant to synthesize:
- N
- -benzylthioureas by reacting with various amines.
- N-benzyl-O-alkyl carbamates by treating with long-chain alcohols.
- 3-mercapto-1,2,4-triazole building block by reacting with formylhydrazine via acyl thiosemicarbazide intermediate formation.
- S-(N-benzylthiocarbamoyl)-L-glutathione and S-(N-benzylthiocarbamoyl)-L-cysteine.
Benzyl isothiocyanate has been used in the preparation of S-(N-benzylthiocarbamoyl)-L-glutathione and S-(N-benzylthiocarbamoyl)-L-cysteine.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Irrit. 2 - Resp. Sens. 1 - Skin Irrit. 2 - Skin Sens. 1 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
222.8 °F
Flash Point(C)
106 °C
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
A Simple and Green Procedure for the Synthesis of N-Benzylthioureas
C de Sequeira Aguiar L, et al.
Letters in Organic Chemistry, 8(8), 540-544 (2011)
W H Mennicke et al.
Xenobiotica; the fate of foreign compounds in biological systems, 18(4), 441-447 (1988-04-01)
1. Both after ingestion of benzyl isothiocyanate (BITC), a compound with antibacterial properties, and after consumption of garden cress known to contain BITC, the metabolite N-acetyl-S-(N-benzylthiocarbamoyl)-L-cysteine was identified in the urine of volunteers by comparative chromatography. 2. The chemical structure
G Brüsewitz et al.
The Biochemical journal, 162(1), 99-107 (1977-01-15)
1. The corresponding cysteine conjugate was formed when the GSH (reduced glutathione) or cysteinylglycine conjugates of benzyl isothiocyanate were incubated with rat liver or kidney homogenates. When the cysteine conjugate of benzyl isothiocyanate was similarly incubated in the presence of
Synthesis of 5-substituted 3-mercapto-1, 2, 4-triazoles via Suzuki-Miyaura reaction
Katkevica S, et al.
Tetrahedron Letters, 54(34), 4524-4525 (2013)
Sanjay K Srivastava et al.
Carcinogenesis, 25(9), 1701-1709 (2004-05-01)
Benzyl isothiocyanate (BITC), a cruciferous vegetable-derived compound, has been shown to inhibit chemically induced cancer in animal models. Moreover, epidemiological studies have provided compelling evidence to suggest that cruciferous vegetables may be protective against cancer risk. Here, we report that
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service