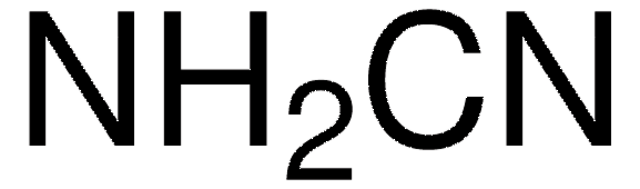All Photos(1)
About This Item
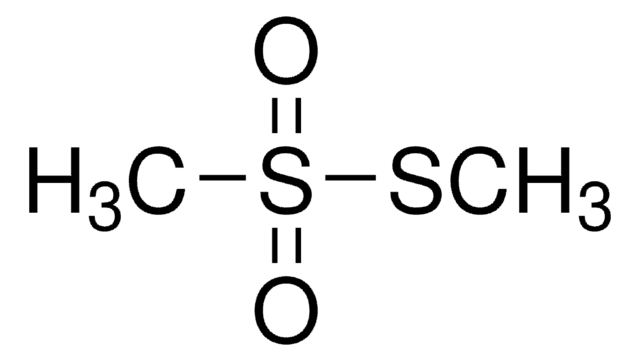
Linear Formula:
(CH3S)2C=NCN
CAS Number:
Molecular Weight:
146.23
Beilstein:
635998
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
90%
form
solid
mp
45-50 °C (lit.)
SMILES string
CS\C(SC)=N\C#N
InChI
1S/C4H6N2S2/c1-7-4(8-2)6-3-5/h1-2H3
InChI key
IULFXBLVJIPESI-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
Dimethyl N-cyanodithioiminocarbonate has been used in the synthesis of:
- 4-methylthiopyrazolo[1,5-a]-1,3,5-triazines
- methylsulfanyl derivatives of azoloazines and azoloazoles
- methylsulfanylpyrimidines
- N-aryl-6-methylsulfanyl-4-oxopyrimidine-5-carbonitriles
- cyanoguanidines
Other Notes
Remainder water
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Lot/Batch Number
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
D Z Hung et al.
Journal of toxicology. Clinical toxicology, 30(3), 351-358 (1992-01-01)
Dimethyl cyanocarbonimidodithioate (CAS No. 10191-60-3) a raw material for cimetidine synthesis, is labelled as an irritant on its storage tank. There is no information available regarding the toxic effects of human exposure. We report a case of severe dermatitis clinically
The design and synthesis of structurally related mercaptopurine analogues: reaction of dimethyl N-cyano-dithioiminocarbonate with 5-aminopyrazoles.
Elgemeie GH, et al.
Synthetic Communications, 31(22), 3453-3458 (2001)
Novel Mercaptopurine and Thioguanine Analogues: The Reaction of Dimethyl N-Cyanodithioiminocarbonate with Oxo-and Amino-diazoles.
Alqaradawi SY and Elgemeie GH.
Synthetic Communications, 34(5), 805-815 (2004)
A novel synthesis of N-aryl-6-methylsulfanyl-4-pyrimidinones and purine analogues: The reaction of dimethyl N-cyanodithioiminocarbonate with cyanoacetanilides.
Elgemeie GH, et al.
Synthetic Communications, 33(12), 2095-2101 (2003)
First Synthesis of N-Substituted Amino and N-Sulfonylaminated Methylthiopyrimidines: Reaction of Dimethyl N-Cyanodithioiminocarbonate With Substituted Hydrazides.
Elgemeie GH and Sood SA.
Synthetic Communications, 36(6), 743-753 (2006)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service