All Photos(1)
About This Item
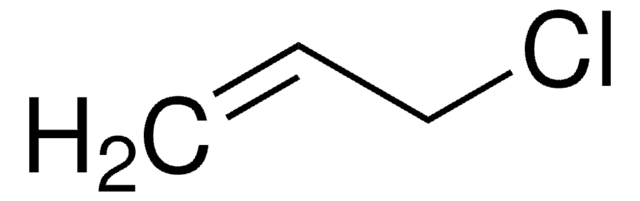
Linear Formula:
ClCH2COOCH=CH2
CAS Number:
Molecular Weight:
120.53
Beilstein:
1745172
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
≥99.0%
contains
~0.01% hydroquinone monomethyl ether as stabilizer
refractive index
n20/D 1.444
density
1.192 g/mL at 20 °C
storage temp.
2-8°C
SMILES string
ClCC(=O)OC=C
InChI
1S/C4H5ClO2/c1-2-7-4(6)3-5/h2H,1,3H2
InChI key
XJELOQYISYPGDX-UHFFFAOYSA-N
Application
Vinyl chloroacetate was used to study the effect of the electron-withdrawing groups on the ligand in a series of bis(acetylacetonate)cobalt(II) derivatives.
Other Notes
Used for the enzyme-catalyzed, regioselective acylation of carbohydrates
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Dam. 1 - Skin Corr. 1B
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Lot/Batch Number
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Radical (co) polymerization of vinyl chloroacetate and N-vinylpyrrolidone mediated by bis (acetylacetonate) cobalt derivatives.
Kaneyoshi H and Matyjaszewski K.
Macromolecules, 39(8), 2757-2763 (2006)
E.W. Holla
Angewandte Chemie (International Edition in English), 101, 222-222 (1989)
Jérémie Brand et al.
Carbohydrate polymers, 169, 189-197 (2017-05-16)
Herein we propose a versatile method for the surface tailoring of cellulose nanocrystals (CNCs) based on the reactivity of vinyl ester molecules toward the accessible hydroxyl groups located at the surface of the nanoparticles. CNCs produced from wood pulp were
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service