243086
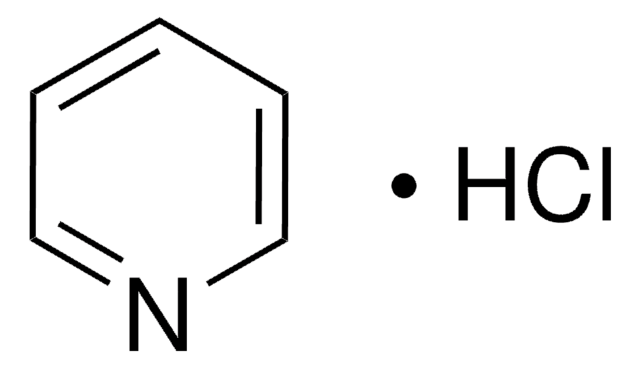
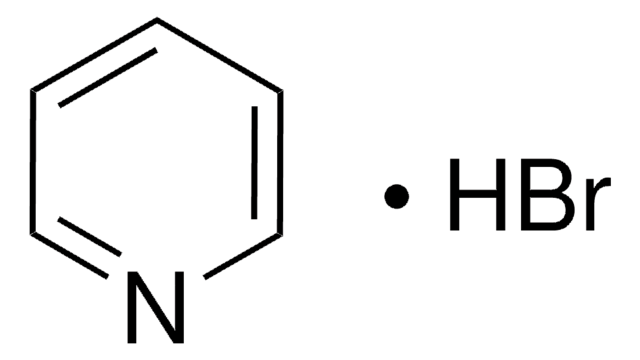
Pyridine hydrochloride
98%
Synonym(s):
Pyridinium chloride
Sign Into View Organizational & Contract Pricing
All Photos(4)
About This Item
Empirical Formula (Hill Notation):
C5H5N · HCl
CAS Number:
Molecular Weight:
115.56
Beilstein:
3615340
EC Number:
MDL number:
UNSPSC Code:
12352100
eCl@ss:
39151701
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
bp
222-224 °C (lit.)
mp
145-147 °C (lit.)
solubility
ethanol: soluble 50 mg/mL, clear, colorless to light yellow
SMILES string
Cl[H].c1ccncc1
InChI
1S/C5H5N.ClH/c1-2-4-6-5-3-1;/h1-5H;1H
InChI key
AOJFQRQNPXYVLM-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Pyridine hydrochloride is an acidic type demethylating agent used as a catalyst in deprotection of aromatic methyl ethers.
Application
Pyridine hydrochloride (pyridine hydrochloride) was used in the demethylation of 4,5-dimethyl-7-methoxy-1-tetralone.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Demethylation of methyl aryl ethers using pyridine hydrochloride in solvent-free conditions under microwave irradiation.
Kulkarni PP, et al.
J. Chem. Res. Synop., 6, 394-395 (1999)
Tatyana N Sevastyanova et al.
Molecular pharmacology, 86(5), 492-504 (2014-08-13)
Metabotropic glutamate receptors (mGluRs) function as dimers. Recent work suggests that mGluR1 and mGluR5 may physically interact, but the nature and functional consequences of this relationship have not been addressed. In this study, the functional and pharmacological consequences of this
Nathalie Ségaud et al.
Inorganic chemistry, 52(2), 691-700 (2013-01-11)
We report the synthesis, characterization, and solution chemistry of a series of new Fe(II) complexes based on the tetradentate ligand N-methyl-N,N'-bis(2-pyridyl-methyl)-1,2-diaminoethane or the pentadentate ones N,N',N'-tris(2-pyridyl-methyl)-1,2-diaminoethane and N,N',N'-tris(2-pyridyl-methyl)-1,3-diaminopropane, modified by propynyl or methoxyphenyltriazolyl groups on the amino functions. Six of
Jipan Yu et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 19(13), 4271-4277 (2013-02-13)
Efficient copper-catalyzed aerobic oxidative C-H and C-C functionalization of 1-[2-(arylamino)aryl]ethanones leading to acridones has been developed. The procedure involves cleavage of aromatic C-H and acetyl C-C bonds with intramolecular formation of a diarylketone bond. The protocol uses inexpensive Cu(O2CCF3)2 as
Ye Wei et al.
Journal of the American Chemical Society, 135(10), 3756-3759 (2013-02-27)
We describe here a [3+3]-type condensation reaction of O-acetyl ketoximes and α,β-unsaturated aldehydes that is synergistically catalyzed by a copper(I) salt and a secondary ammonium salt (or amine). This redox-neutral reaction allows modular synthesis of a variety of substituted pyridines
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service



![2-Mesityl-5-methylimidazo[1,5-a]pyridinium chloride 97%](/deepweb/assets/sigmaaldrich/product/structures/495/055/5d86d2cc-b538-4586-9e2c-9e0d870826a7/640/5d86d2cc-b538-4586-9e2c-9e0d870826a7.png)