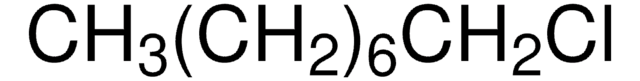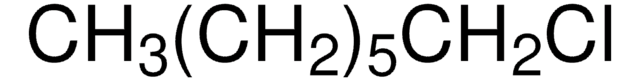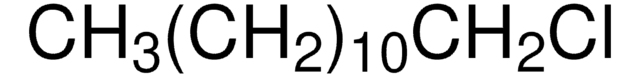All Photos(1)
About This Item
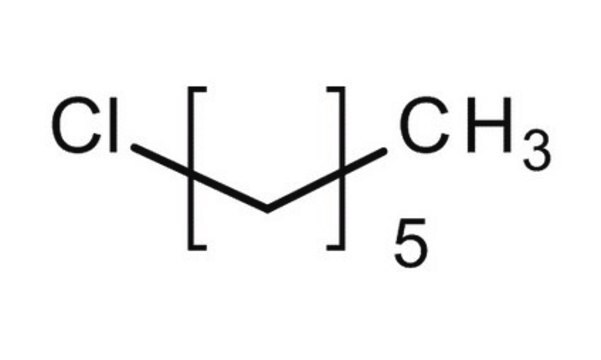
Linear Formula:
CH3(CH2)5Cl
CAS Number:
Molecular Weight:
120.62
Beilstein:
1731289
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
99%
form
liquid
refractive index
n20/D 1.419 (lit.)
bp
133-134 °C (lit.)
mp
−94 °C (lit.)
solubility
water: insoluble(lit.)
density
0.879 g/mL at 25 °C (lit.)
SMILES string
CCCCCCCl
InChI
1S/C6H13Cl/c1-2-3-4-5-6-7/h2-6H2,1H3
InChI key
MLRVZFYXUZQSRU-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Hydrodechlorination of vapours of chlorobenzene and 1-chlorohexane using Ni, Fe, W, Ni-Mo, Pt and Pd on activated carbon or on Al2O3 (catalyst) has been studied. Titanium silicalite catalyzed oxidation of 1-chlorohexane with hydrogen peroxide has been studied.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Flam. Liq. 3
Storage Class Code
3 - Flammable liquids
WGK
WGK 1
Flash Point(F)
80.6 °F - closed cup
Flash Point(C)
27 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Stephen E Repper et al.
Dalton transactions (Cambridge, England : 2003), 46(9), 2821-2828 (2017-02-09)
Absorption of carbon monoxide by copper(i)-containing ionic liquids, [C
Performance of supported nickel and other metal catalysts in the hydrodechlorination of chlorobenzene and 1-chlorohexane.
de Jong V and Louw R.
Applied Catalysis A: General, 271(1), 153-163 (2004)
Horng-Jang Liaw et al.
Journal of hazardous materials, 367, 407-417 (2019-01-06)
Industrial use of ionic liquids may require exposure to high temperatures. We demonstrate that such applications may result in an increase in flammability hazard due to chemical decomposition. The ionic liquid, 1-hexyl-3-methylimidazolium chloride ([C6mim][Cl]), was selected as the study sample.
Oxidation of saturated hydrocarbons with hydrogen peroxide, catalysed by titanium silicalite.
G Clerici M.
Applied Catalysis, 68(1), 249-261 (1999)
Stanislav Mazurenko et al.
PloS one, 13(6), e0198913-e0198913 (2018-06-19)
Analytical devices that combine sensitive biological component with a physicochemical detector hold a great potential for various applications, e.g., environmental monitoring, food analysis or medical diagnostics. Continuous efforts to develop inexpensive sensitive biodevices for detecting target substances typically focus on
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service