232564
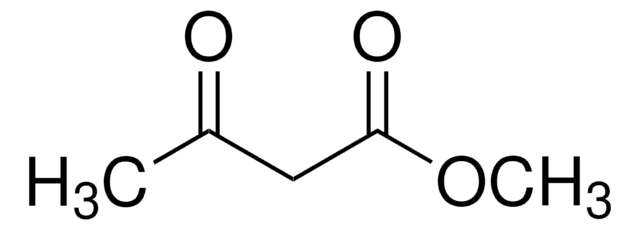
Ethyl 2,4-dioxovalerate
97%
Synonym(s):
Ethyl acetonoxalate
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
CH3COCH2COCOOC2H5
CAS Number:
Molecular Weight:
158.15
Beilstein:
607062
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
refractive index
n20/D 1.474 (lit.)
bp
101-103 °C/12 mmHg (lit.)
mp
16-18 °C (lit.)
density
1.126 g/mL at 25 °C (lit.)
functional group
ester
ketone
storage temp.
2-8°C
SMILES string
CCOC(=O)C(=O)CC(C)=O
InChI
1S/C7H10O4/c1-3-11-7(10)6(9)4-5(2)8/h3-4H2,1-2H3
InChI key
OYQVQWIASIXXRT-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Assymmmetric hydrogenation of ethyl 2,4-dioxovalerate in the presence of chiral rhodium or ruthenium catalysts yields 2-hydroxy-4-methyltetrahydrofuran-2-one. Ethyl 2,4-dioxovalerate is a potential anti-fungal agent.
Application
Ethyl 2,4-dioxovalerate was used in the preparation of:
- 1H-pyrazolo-[3,4-d]-pyridazin-7(6H)-one core analog
- pyrazole
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
235.4 °F - closed cup
Flash Point(C)
113 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Acylpyruvates as potential antifungal agents.
H A Burch
Journal of medicinal chemistry, 15(4), 429-431 (1972-04-01)
New one pot synthesis of a chiral a-hydroxy-?-butyrolactone via sequential asymmetric hydrogenation of an a, ?-diketoester.
Blandin V, et al.
Tetrahedron Asymmetry, 9(16), 2765-2768 (1998)
Stephen C McKeown et al.
Bioorganic & medicinal chemistry letters, 16(18), 4767-4771 (2006-07-18)
The discovery, synthesis and structure-activity relationship (SAR) of a novel series of EP1 receptor antagonists is described. Pyrazole acid 4, identified from a chemical array, had desirable physicochemical properties, an excellent in vitro microsomal inhibition and cytochrome P450 (CYP450) profile
John M Fevig et al.
Bioorganic & medicinal chemistry letters, 16(14), 3755-3760 (2006-05-10)
Previously, potent factor Xa inhibitors were described based on a pyrazole core. Modifications of the pyrazole core have provided additional novel, highly potent factor Xa inhibitors. This manuscript will describe the synthesis and biological activity of factor Xa inhibitors containing
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service