All Photos(2)
About This Item
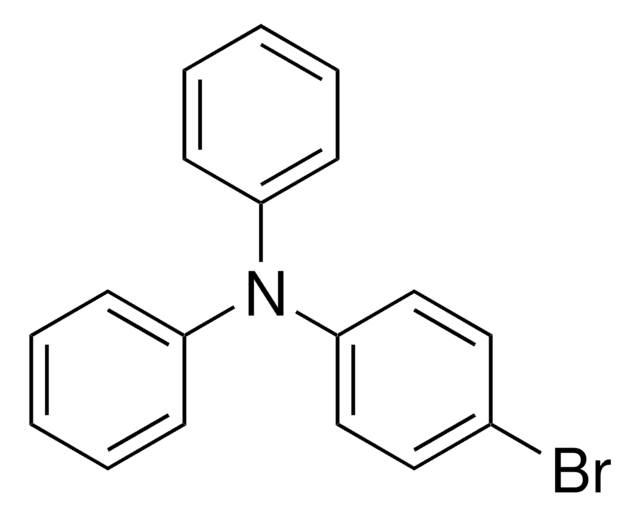
Linear Formula:
(BrC6H4)3N
CAS Number:
Molecular Weight:
482.01
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
mp
141-143 °C (lit.)
SMILES string
Brc1ccc(cc1)N(c2ccc(Br)cc2)c3ccc(Br)cc3
InChI
1S/C18H12Br3N/c19-13-1-7-16(8-2-13)22(17-9-3-14(20)4-10-17)18-11-5-15(21)6-12-18/h1-12H
InChI key
ZRXVCYGHAUGABY-UHFFFAOYSA-N
Application
Tris(4-bromophenyl)amine was used in the synthesis of porous luminescent covalent--organic polymers (COPs).
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Zhonghua Xiang et al.
Macromolecular rapid communications, 33(14), 1184-1190 (2012-04-18)
Three porous luminescent covalent--organic polymers (COPs) have been synthesized through self-polycondensation of the monomers of tris(4-bromophenyl)amine, 1,3,5-tris(4-bromophenyl)benzene, and 2,4,6-tris-(4-bromo-phenyl)-[1,3,5]triazine by using Ni-catalyzed Yamamoto reaction. All the COP materials possess not only high Brunauer-Emmett-Teller (BET) specific surface area of about 2000
S A Ponomarenko et al.
Faraday discussions, 174, 313-339 (2014-10-04)
This contribution describes recent progress in the design, synthesis and properties of solution-processible star-shaped oligomers and their application in organic photovoltaics. Even though alternative chemistry has been used to design such oligomers, the most successful approach is based on a
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service

![4,4′,4′′-Tris[phenyl(m-tolyl)amino]triphenylamine 98.0%](/deepweb/assets/sigmaaldrich/product/structures/370/101/1022653f-ed3b-4991-a82c-269ad710b908/640/1022653f-ed3b-4991-a82c-269ad710b908.png)