227781
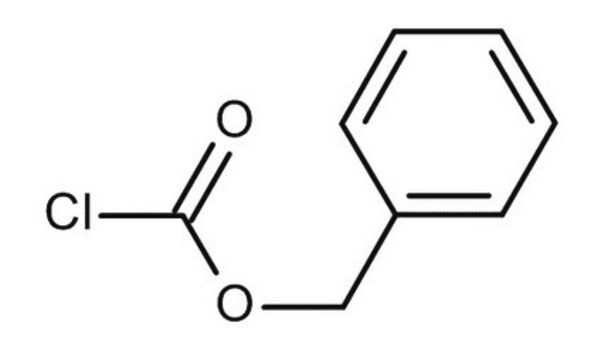
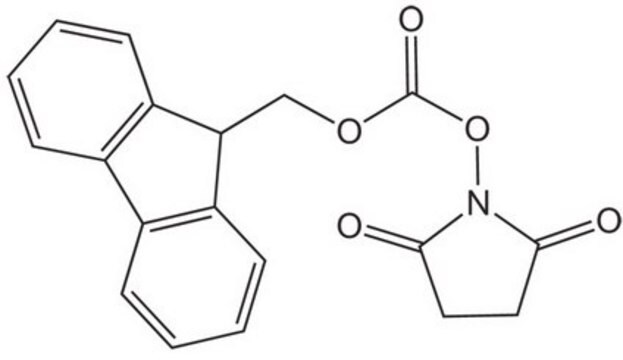
N-(Benzyloxycarbonyloxy)succinimide
98%, for peptide synthesis
Synonym(s):
Benzyl N-succinimidyl carbonate, Z-OSu
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Empirical Formula (Hill Notation):
C12H11NO5
CAS Number:
Molecular Weight:
249.22
Beilstein:
1387927
EC Number:
MDL number:
UNSPSC Code:
12352005
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
product name
N-(Benzyloxycarbonyloxy)succinimide, 98%
Quality Level
Assay
98%
mp
80-82 °C (lit.)
application(s)
peptide synthesis
SMILES string
O=C(OCc1ccccc1)ON2C(=O)CCC2=O
InChI
1S/C12H11NO5/c14-10-6-7-11(15)13(10)18-12(16)17-8-9-4-2-1-3-5-9/h1-5H,6-8H2
InChI key
MJSHDCCLFGOEIK-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
N-(Benzyloxycarbonyloxy)succinimide (Cbz-OSu) is a common reagent for the carboxybenzyl protection of amines. This reaction is one of the key synthetic steps in the synthesis of:
Cbz-OSu is widely employed to protect amino acid residues in peptide synthesis. It can also be used in N-trans diprotection of cyclen regioselectively.
- Enantiomers of cyclic methionine analogs viz, (R)-and (S)-3-aminotetrahydrothiophene- 3-carboxylic acid.
- 1′-H-Spiro-(indoline-3,4′-piperidine) and its derivatives.
- Total synthesis of (-)-diazonamide A. and (-)-sanglifehrin A.
Cbz-OSu is widely employed to protect amino acid residues in peptide synthesis. It can also be used in N-trans diprotection of cyclen regioselectively.
Reagent for the selective introduction of the Z-amino protection in amino acids; and in aminoglycoside antibiotics.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
A general asymmetric synthesis of syn-and anti-β-substituted cysteine and serine derivatives.
Xiong C, et al.
The Journal of Organic Chemistry, 67(10), 3514-3517 (2002)
Highly regioselective N-trans symmetrical diprotection of cyclen
De Leon-Rodriguez LM, et al.
Tetrahedron Letters, 47(39), 6937-6940 (2006)
Reconstitution of peptidoglycan cross-linking leads to improved fluorescent probes of cell wall synthesis.
Lebar M D, et al.
Journal of the American Chemical Society, 136(31), 10874-10877 (2014)
A convergent three-component total synthesis of the powerful immunosuppressant (−)-sanglifehrin A.
Paquette L A, et al.
Journal of the American Chemical Society, 124(16), 4257-4270 (2002)
Pd/C (en)-Catalyzed chemoselective hydrogenation with retention of the N-Cbz protective group and its scope and limitations.
Hattori K, et al.
Tetrahedron, 56(43), 8433-8441 (2000)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service