All Photos(2)
About This Item
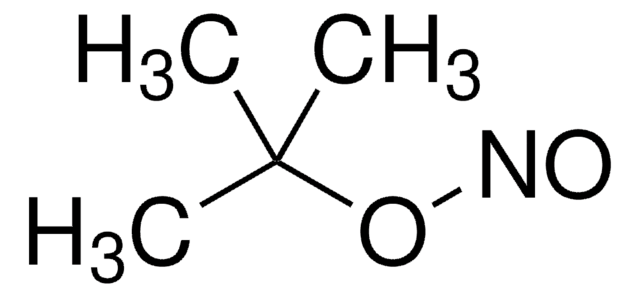
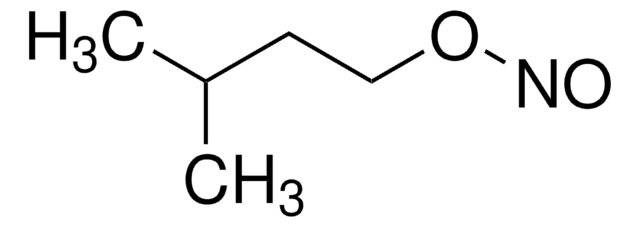
Linear Formula:
CH3(CH2)3ONO
CAS Number:
Molecular Weight:
103.12
Beilstein:
1701036
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
vapor pressure
760 mmHg ( 78 °C)
Quality Level
Assay
95%
form
liquid
refractive index
n20/D 1.376 (lit.)
bp
78 °C (lit.)
solubility
alcohol: miscible(lit.)
diethyl ether: miscible(lit.)
density
0.882 g/mL at 25 °C (lit.)
storage temp.
2-8°C
SMILES string
CCCCON=O
InChI
1S/C4H9NO2/c1-2-3-4-7-5-6/h2-4H2,1H3
InChI key
JQJPBYFTQAANLE-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
The photodissociation dynamics of butyl nitrite was studied using time-resolved Fourier transform infrared (TR-FTIR) emission spectroscopy. The effects of butyl nitrite on methyl cobalamin and 5-methyl tetrahydrofolate were studied.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 2 Inhalation - Acute Tox. 3 Oral - Flam. Liq. 2
Storage Class Code
3 - Flammable liquids
WGK
WGK 1
Flash Point(F)
8.6 °F - closed cup
Flash Point(C)
-13 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
G R Newell et al.
Pharmacotherapy, 4(5), 284-291 (1984-09-01)
Volatile nitrite in the form of amyl nitrite was used for 100 years for the treatment of angina pectoris. In spite of recognized toxicity, its use in this form was considered safe. During the 1960s prescriptions were not required for
J D Osterloh et al.
Journal of pharmaceutical sciences, 74(7), 780-782 (1985-07-01)
The uptake of butyl nitrite by rats (500 g, one rat/chamber) was determined over a 5-min exposure period. About 44% of the starting amount (771-3855 ppm) of n-butyl nitrite was consumed in 5 min. Three rats per exposure concentration were
Tsuyoshi Taniguchi et al.
Chemical communications (Cambridge, England), 49(22), 2198-2200 (2013-02-12)
A method for direct functionalization of three positions including an unactivated C-H bond of aliphatic alkenes using tert-butyl nitrite and molecular oxygen to give γ-lactols has been developed. The present reaction proceeds through a sequence of radical processes involving oxynitration
Dipankar Koley et al.
Organic letters, 11(18), 4172-4175 (2009-08-25)
tert-Butyl nitrite was identified as a safe and chemoselective nitrating agent that provides preferentially mononitro derivatives of phenolic substrates in the presence of potentially competitive functional groups. On the basis of our control experiments, we propose that the reaction proceeds
E Roth et al.
American journal of hematology, 20(2), 153-159 (1985-10-01)
The use of volatile butylnitrite in place of sodium nitrite for the in vitro production of methemoglobin was explored in studies of G6PD-deficient red cells and for measurements of the red cell methemoglobin reductase activity. It was found that butylnitrite
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service