All Photos(3)
About This Item
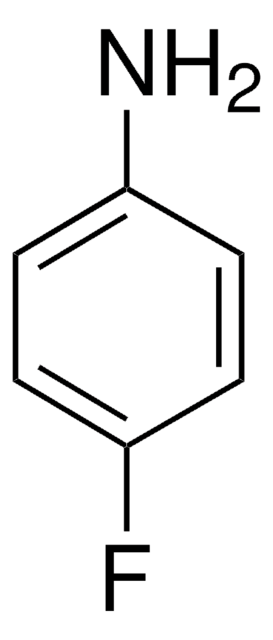
Linear Formula:
FC6H4NHCH3
CAS Number:
Molecular Weight:
125.14
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
refractive index
n20/D 1.5320 (lit.)
bp
79 °C/11 mmHg (lit.)
density
1.040 g/mL at 25 °C (lit.)
SMILES string
CNc1ccc(F)cc1
InChI
1S/C7H8FN/c1-9-7-4-2-6(8)3-5-7/h2-5,9H,1H3
InChI key
VLWRKVBQUANIGI-UHFFFAOYSA-N
Application
4-Fluoro-N-methylaniline was used as a model compound to study the in vivo and in vitro biotransformation of secondary aromatic amines.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 3 Oral - Eye Dam. 1 - Skin Irrit. 2 - Skin Sens. 1 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 2
Flash Point(F)
168.8 °F - closed cup
Flash Point(C)
76 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
James P Driscoll et al.
Chemical research in toxicology, 23(5), 861-863 (2010-04-08)
Here, we report on the mechanism by which flavin-containing monooxygenase 1 (FMO1) mediates the formation of a reactive intermediate of 4-fluoro-N-methylaniline. FMO1 catalyzed a carbon oxidation reaction coupled with defluorination that led to the formation of 4-N-methylaminophenol, which was a
M G Boersma et al.
Drug metabolism and disposition: the biological fate of chemicals, 21(2), 218-230 (1993-03-01)
In vivo and in vitro biotransformation of secondary aromatic amines was investigated using 4-fluoro-N-methylaniline as the model compound. Attention was focused on the role of cytochromes P-450 and the flavin-containing monooxygenase in formation of the various metabolic products. In vitro
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service