All Photos(1)
About This Item
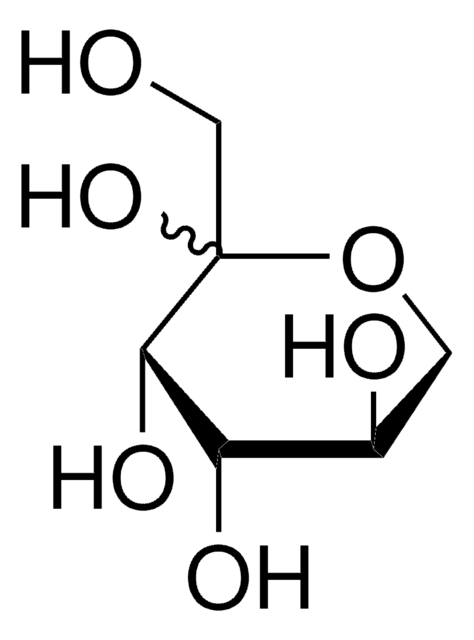
Empirical Formula (Hill Notation):
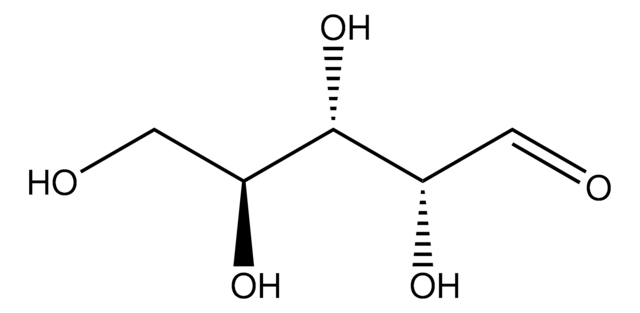
C5H10O5
CAS Number:
Molecular Weight:
150.13
Beilstein:
1723083
EC Number:
MDL number:
UNSPSC Code:
12352201
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
99%
optical activity
[α]25/D -15.8°, c = 4 in H2O
mp
108-112 °C (lit.)
SMILES string
O[C@@H]1COC(O)[C@@H](O)[C@H]1O
InChI
1S/C5H10O5/c6-2-1-10-5(9)4(8)3(2)7/h2-9H,1H2/t2-,3+,4+,5?/m1/s1
InChI key
SRBFZHDQGSBBOR-AGQMPKSLSA-N
Related Categories
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Álvaro Vázquez-Mayagoitia et al.
Astrobiology, 11(2), 115-121 (2011-03-12)
The RNA-world theory hypothesizes that early Earth life was based on the RNA molecule. However, the notion that ribose, the sugar in RNA, is unstable still casts a serious doubt over this theory. Recently, it has been found that the
Carsten Hofmann et al.
Chemistry & biology, 12(10), 1137-1143 (2005-10-26)
The oligosaccharide antibiotic avilamycin A is composed of a polyketide-derived dichloroisoeverninic acid moiety attached to a heptasaccharide chain consisting of six hexoses and one unusual pentose moiety. We describe the generation of mutant strains of the avilamycin producer defective in
M Jørgensen et al.
The Journal of organic chemistry, 66(13), 4625-4629 (2001-06-26)
The combination of a Wittig olefination and a dihydroxylation reaction constitutes a facile synthetic protocol for the transformation of unprotected carbohydrates into higher sugars. The Wittig reaction is carried out with tert-butyl or diphenylmethyl ester stabilized phosphoranes to give (E)-configured
Michal Letek et al.
PLoS genetics, 6(9), e1001145-e1001145 (2010-10-14)
We report the genome of the facultative intracellular parasite Rhodococcus equi, the only animal pathogen within the biotechnologically important actinobacterial genus Rhodococcus. The 5.0-Mb R. equi 103S genome is significantly smaller than those of environmental rhodococci. This is due to
M Beier et al.
Science (New York, N.Y.), 283(5402), 699-703 (1999-01-29)
All four members of the family of pentopyranosyl-(2'-->4') oligonucleotide systems that contain beta-ribo-, beta-xylo-, alpha-lyxo-, or alpha-arabinopyranosyl units as repeating sugar building blocks are found to be much stronger Watson-Crick base-pairing systems than RNA. The alpha-arabinopyranosyl system is the strongest
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service