21860
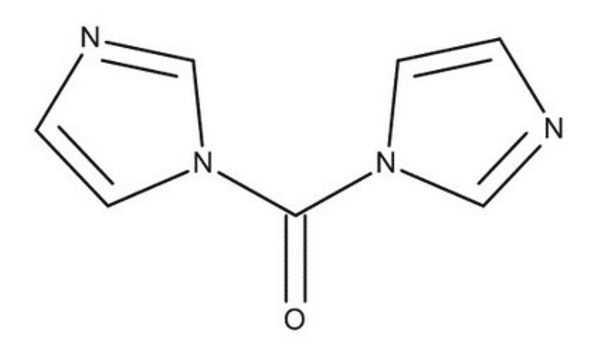
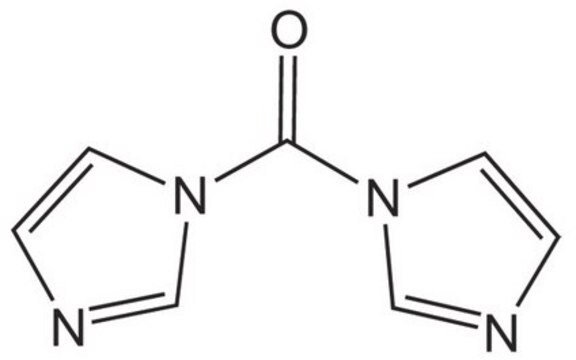
CDI
≥97.0% (T), for peptide synthesis
Synonym(s):
1,1′-Carbonyldiimidazole
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C7H6N4O
CAS Number:
Molecular Weight:
162.15
Beilstein:
6826
EC Number:
MDL number:
UNSPSC Code:
12352115
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
product name
CDI, ≥97.0% (T)
Quality Level
Assay
≥97.0% (T)
form
solid
reaction suitability
reaction type: Carbonylations
mp
115-122 °C
117-122 °C (lit.)
application(s)
peptide synthesis
storage temp.
2-8°C
SMILES string
O=C(n1ccnc1)n2ccnc2
InChI
1S/C7H6N4O/c12-7(10-3-1-8-5-10)11-4-2-9-6-11/h1-6H
InChI key
PFKFTWBEEFSNDU-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
CDI serves as a powerful coupling reagent in peptide synthesis and organic chemistry.
Application
CDI (1,1′-Carbonyldiimidazole) can be used:
- In the activation of malonic acid derivatives to prepare carbonyl imidazoles by mild decarboxylation.
- In the activation of cellulose membranes for the development of immunosensors.
- In the synthesis of substituted 1,3-oxazolo[4,5-d] pyridazinones.
- As a reagent for the conversion of various hydroxamic acids to isocyanates by Lossen rearrangement.
- In the synthesis of 1,3,4-oxadiazole derivatives , and peptide thioesters in water.
- As an acylation agent for the synthesis of carbamates.
Other Notes
Reactive reagent for the activation of acids as imidazolides: synthesis of esters, amides, ketones etc. Reagent for immobilizing enzymes and affinity ligands; Carbonyl-transfer reagent for the synthesis of various heterocycles, some recent applications
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Dam. 1 - Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 2
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
J. Barluenga et al.
The Journal of Organic Chemistry, 56, 6751-6751 (1991)
Mechanochemical 1, 1′-Carbonyldiimidazole-Mediated Synthesis of Carbamates
Lanzillotto M, et al.
ACS sustainable chemistry & engineering, 11(3), 2882-2889 (2015)
An efficient synthesis of novel 1, 3-oxazolo [4, 5-d] pyridazinones
Frolov EB, et al.
Tetrahedron Letters, 45(24), 4693-4696 (2004)
Activation of cellulose membranes with 1, 1′-carbonyldiimidazole or 1-cyano-4-dimethylaminopyridinium tetrafluoroborate as a basis for the development of immunosensors
Stollner, D, et al.
Analytical Biochemistry, 2(304), 157-165 (2002)
Mild Decarboxylative Activation of Malonic Acid Derivatives by 1, 1′-Carbonyldiimidazole
Lafrance D, et al.
Organic Letters, 9(13), 2322-2325 (2011)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service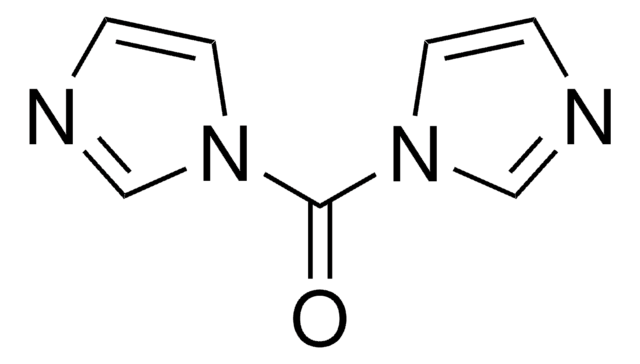


![1,8-Diazabicyclo[5.4.0]undec-7-ene 98%](/deepweb/assets/sigmaaldrich/product/structures/120/564/5b373e23-1624-489c-8efb-692de0f96ffb/640/5b373e23-1624-489c-8efb-692de0f96ffb.png)