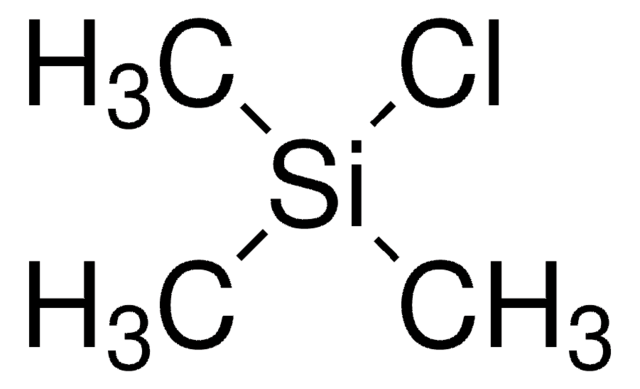205354
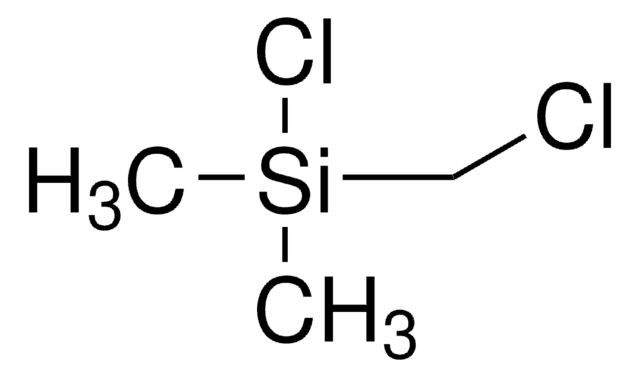
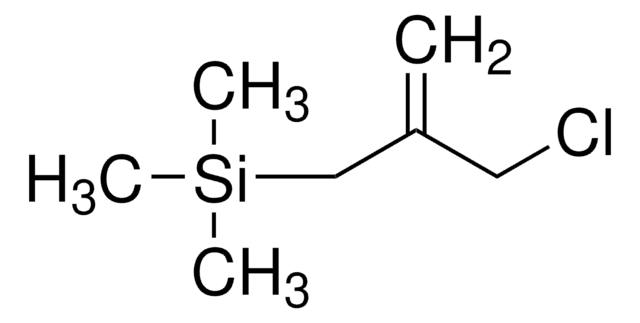
(Chloromethyl)trimethylsilane
98%
Synonym(s):
(Trimethylsilyl)methyl chloride
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
(CH3)3SiCH2Cl
CAS Number:
Molecular Weight:
122.67
Beilstein:
906705
EC Number:
MDL number:
UNSPSC Code:
12352103
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
vapor pressure
~25 mmHg ( 20 °C)
Quality Level
Assay
98%
form
liquid
refractive index
n20/D 1.418 (lit.)
bp
98-99 °C (lit.)
density
0.879 g/mL at 25 °C (lit.)
SMILES string
C[Si](C)(C)CCl
InChI
1S/C4H11ClSi/c1-6(2,3)4-5/h4H2,1-3H3
InChI key
OOCUOKHIVGWCTJ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
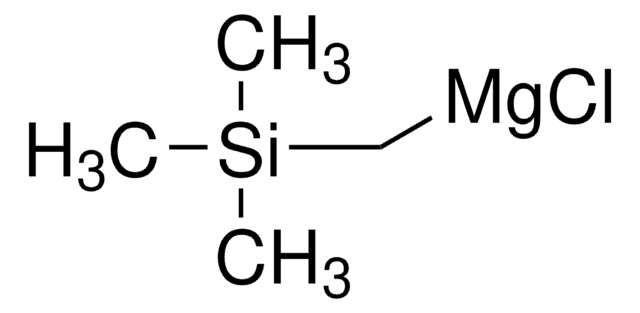
(Chloromethyl)trimethylsilane can be treated with aldehydes or ketones in the presence of triphenylphosphine to synthesize terminal alkenes. It can also be used in the preparation of a reagent, trimethylsilylmethyl magnesium chloride, commonly used in Peterson methylenation.
Packaging
The 25 mL Sure/Seal™ bottle is recommended as a single-use bottle. Repeated punctures will likely result in decreased performance of product.
Legal Information
Sure/Seal is a trademark of Sigma-Aldrich Co. LLC
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Flam. Liq. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
24.8 °F - closed cup
Flash Point(C)
-4 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Direct synthesis of terminal olefins from ketones. Application of (chloromethyl) trimethylsilane to a Wittig reaction.
Sekiguchi A and Ando W
The Journal of Organic Chemistry, 44(3), 413-415 (1979)
Synthesis of alkenes from carbonyl compounds and carbanions alpha to silicon. III. Full report and a synthesis of the sex pheromone of gypsy moth.
Chan T H and Chang E
The Journal of Organic Chemistry, 39(22), 3264-3268 (1974)
Methylenation of Perfluoroalkyl Ketones using a Peterson Olefination Approach.
Hamlin T A, et al.
The Journal of Organic Chemistry, 79(3), 1145-1155 (2014)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service