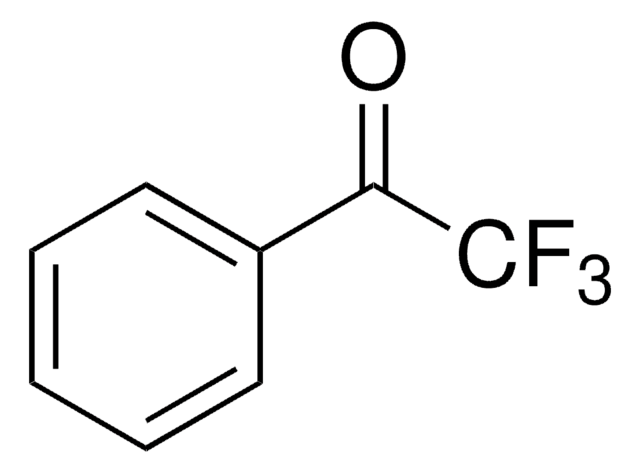
196681
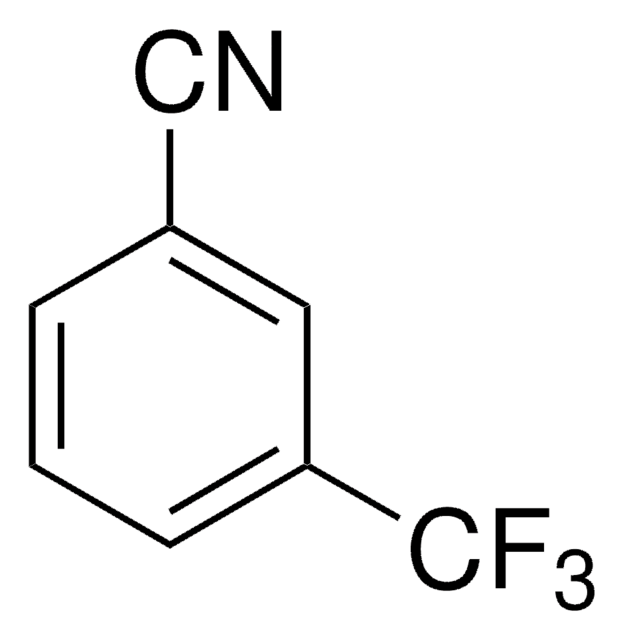
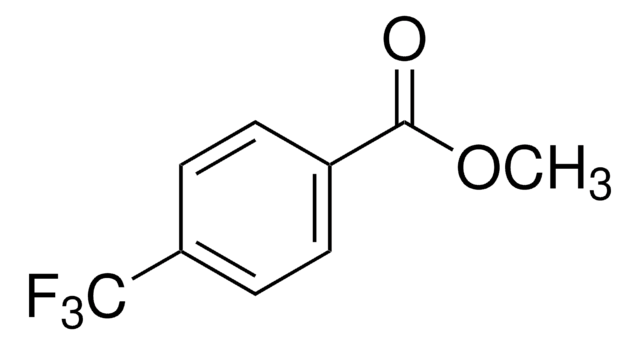
4-(Trifluoromethyl)benzonitrile
99%
Synonym(s):
α,α,α-Trifluoro-p-tolunitrile
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
CF3C6H4CN
CAS Number:
Molecular Weight:
171.12
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
99%
form
solid
refractive index
n20/D 1.4583 (lit.)
bp
80-81 °C/20 mmHg (lit.)
mp
39-41 °C (lit.)
density
1.278 g/mL at 25 °C (lit.)
SMILES string
FC(F)(F)c1ccc(cc1)C#N
InChI
1S/C8H4F3N/c9-8(10,11)7-3-1-6(5-12)2-4-7/h1-4H
InChI key
DRNJIKRLQJRKMM-UHFFFAOYSA-N
General description
4-(Trifluoromethyl)benzonitrile is a key intermediate in the synthesis of fluvoxamine. It participates in nickel-catalyzed arylcyanation reaction of 4-octyne.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Flam. Sol. 1
Storage Class Code
4.1B - Flammable solid hazardous materials
WGK
WGK 3
Flash Point(F)
161.6 °F - closed cup
Flash Point(C)
72 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Yasuhiro Hirata et al.
Journal of the American Chemical Society, 131(31), 10964-10973 (2009-09-03)
Allyl cyanides are found to add across alkynes in the presence of a nickel/P(4-CF(3)-C(6)H(4))(3) catalyst to give polysubstituted 2,5-hexadienenitriles with defined stereo- and regiochemistry. Use of AlMe(2)Cl or AlMe(3) as a Lewis acid cocatalyst accelerates the reaction and expands the
Improving palladium-catalyzed cyanation of aryl halides: development of a state-of-the-art methodology using potassium hexacyanoferrate (II) as cyanating agent.
Schareina T, et al.
Journal of Organometallic Chemistry, 689(24), 4576-4583 (2004)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service