196320
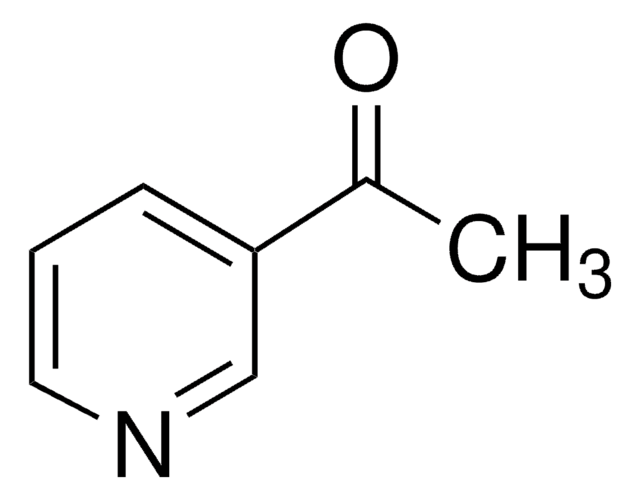
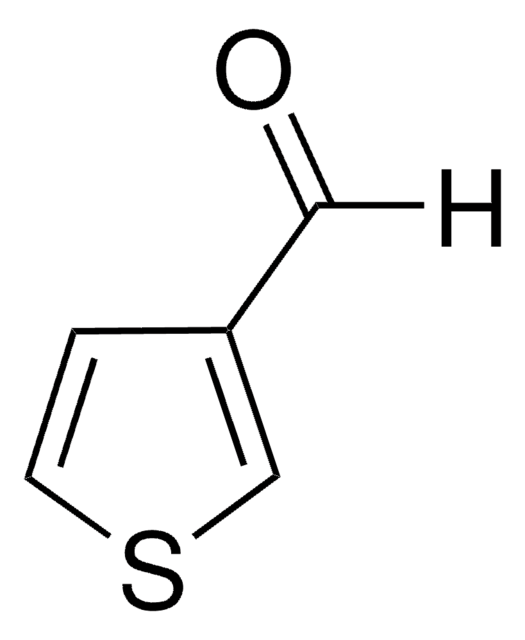
3-Acetylthiophene
98%
Synonym(s):
Methyl-3-thienyl ketone
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C6H6OS
CAS Number:
Molecular Weight:
126.18
Beilstein:
107241
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
form
solid
bp
208-210 °C/748 mmHg (lit.)
mp
57-62 °C (lit.)
SMILES string
CC(=O)c1ccsc1
InChI
1S/C6H6OS/c1-5(7)6-2-3-8-4-6/h2-4H,1H3
InChI key
RNIDWJDZNNVFDY-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
3-Acetylthiophene modified glassy carbon electrode has been used in voltammetric determination of uric acid in urine samples.
Application
3-Acetylthiophene was used in the preparation of 1-(methylthiophenylidine)-8-naphthylamine(Schiff′s base) and 1-(thiophen-3-yl)ethanone.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Electro-oxidative polymerization of Schiff-base of 1, 8-diaminonaphthaline and 3-acetylthiophene. I. Preparation and study the redox behaviour of the resulting polymer.
Hathoot AA.
Eur. Polymer J., 36(5), 1063-1071 (2000)
Ewa Rozycka-Sokolowska et al.
Acta crystallographica. Section C, Crystal structure communications, 67(Pt 6), o209-o211 (2011-06-03)
The structure of the title compound, C(6)H(6)OS, exhibits a flip-type disorder of the thiophene ring [occupancy ratio = 0.848 (3):0.152 (3)], which is typical for many thiophene derivatives. The puckered thiophene ring is essentially coplanar with the plane formed by
Mehmet Aslanoglu et al.
Chemical & pharmaceutical bulletin, 56(3), 282-286 (2008-03-04)
A reliable and reproducible method for the determination of uric acid in urine samples has been developed. The method is based on the modification of a glassy carbon electrode by 3-acetylthiophene using cyclic voltammetry. The poly(3-acetylthiophene) modified glassy carbon electrode
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service