All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C6H8N2
CAS Number:
Molecular Weight:
108.14
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
form
solid
bp
100 °C/0.1 mmHg (lit.)
mp
124-125 °C (lit.)
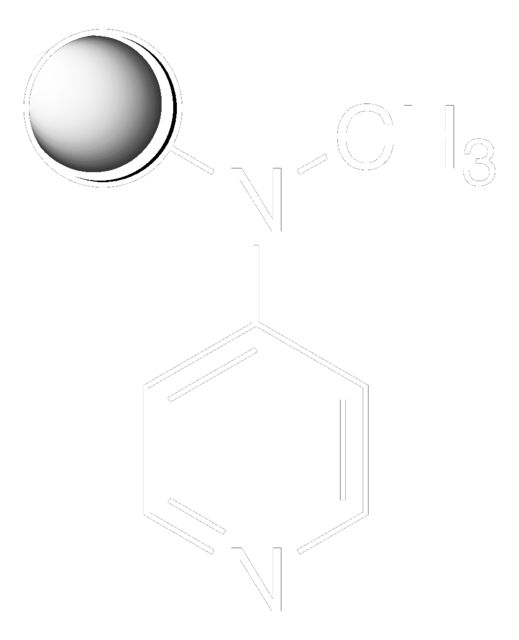
SMILES string
CNc1ccncc1
InChI
1S/C6H8N2/c1-7-6-2-4-8-5-3-6/h2-5H,1H3,(H,7,8)
InChI key
LSCYTCMNCWMCQE-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
4-(Methylamino)pyridine can be used:
- To functionalize hypercrosslinked emulsion-templated porous polymers (polyHIPE) to form a highly efficient heterogeneous nucleophilic catalyst for the acylation of a tertiary alcohol.
- As a reactant to synthesize 4-(N-allyl-N-methylamino)pyridine, which is employed as an intermediate to prepare DMAP/SBA-15 supported catalyst for the synthesis of propylene carbonate.
- To prepare 5-azaoxindoles via homolytic aromatic substitution.
4-(Methylamino)pyridine was employed as efficient nucleophilic catalyst during the preparation of ultra-high surface area emulsion templated porous polymers.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Ultra-high surface area functional porous polymers by emulsion templating and hypercrosslinking: efficient nucleophilic catalyst supports.
Irena Pulko et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 16(8), 2350-2354 (2010-01-28)
Y Amaki et al.
Masui. The Japanese journal of anesthesiology, 38(5), 661-665 (1989-05-01)
A new muscle relaxant antagonist, 4-aminopyridine (4-AMP), has various problems related to its side effects. In order to obtain 4-AMP derivatives with less side effect and the same antagonizing effect on dTc block as that of 4-AMP, three types of
Ultra-high surface area functional porous polymers by emulsion templating and hypercrosslinking: efficient nucleophilic catalyst supports
Pulko I, et al.
Chemistry?A European Journal , 16(8), 2350-2354 (2010)
Homolytic aromatic substitution: A radical approach towards the synthesis of 5-azaoxindoles
Storey J MD and Ladwa MM
Tetrahedron Letters, 47(3), 381-383 (2006)
Tobias Rogosch et al.
Bioorganic & medicinal chemistry, 20(1), 101-107 (2011-12-17)
Dipyrone is a common antipyretic drug and the most popular non-opioid analgesic in many countries. In spite of its long and widespread use, molecular details of its fate in the body are not fully known. We administered dipyrone orally to
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service