193453
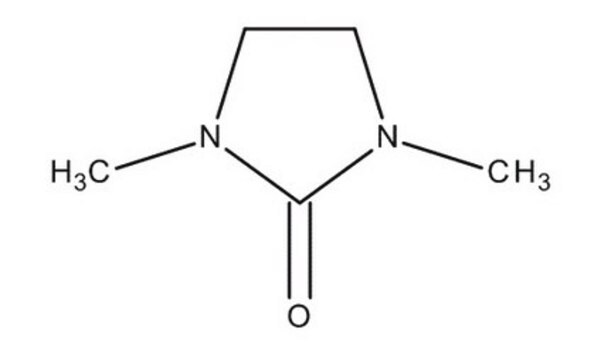
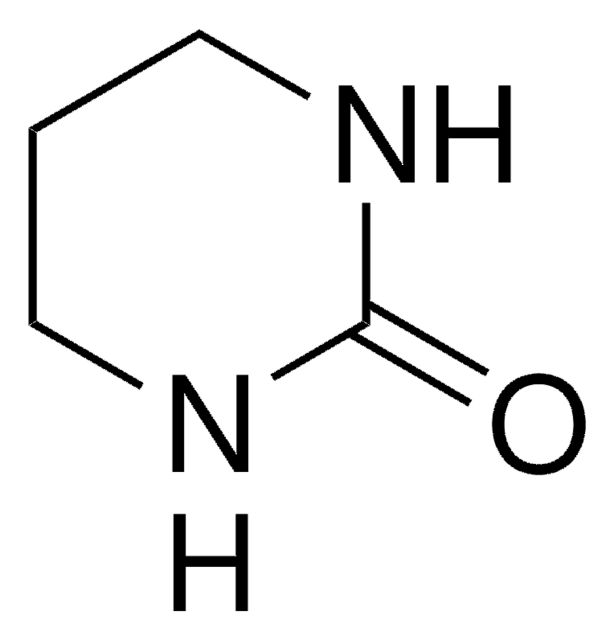
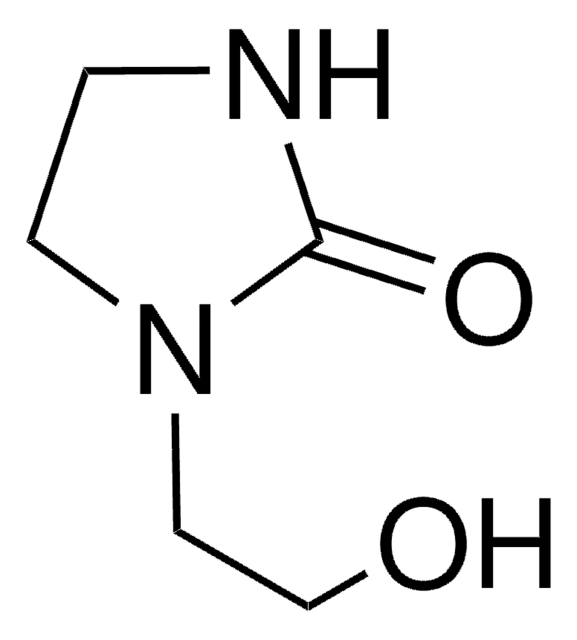
1,3-Dimethyl-2-imidazolidinone
reagent grade
Synonym(s):
N,N′-Dimethylethyleneurea, DMEU, DMI
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C5H10N2O
CAS Number:
Molecular Weight:
114.15
Beilstein:
108808
EC Number:
MDL number:
UNSPSC Code:
12352005
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
grade
reagent grade
Quality Level
form
liquid
refractive index
n20/D 1.472 (lit.)
bp
224-226 °C (lit.)
solubility
toluene: soluble(lit.)
water: miscible
density
1.056 g/mL at 25 °C (lit.)
SMILES string
CN1CCN(C)C1=O
InChI
1S/C5H10N2O/c1-6-3-4-7(2)5(6)8/h3-4H2,1-2H3
InChI key
CYSGHNMQYZDMIA-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
1,3-Dimethyl-2-imidazolidinone constitutes the mobile phase during size-exclusion chromatographic analysis of cellulose.
Application
1,3-Dimethyl-2-imidazolidinone was used as substitute solvent for toxic HMPA in the synthesis of 1,2-bis(trimethylsilyl)benzene. It was employed as solvent during α-regioselective prenylation of imines.
Other Notes
Solvent used in various synthetic organic transformations. Studied in the formation of functionally stabilized hydrosilanediyl-transition metal complexes produced photochemically from arylsilanes.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Dam. 1 - Repr. 2
Storage Class Code
10 - Combustible liquids
WGK
WGK 2
Flash Point(F)
219.2 °F - closed cup
Flash Point(C)
104 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
SEC-MALLS analysis of cellulose using LiCl/1, 3-dimethyl-2-imidazolidinone as an eluent.
Yanagisawa M, et al.
Cellulose, 11(2), 169-176 (2004)
Tsugio Kitamura et al.
The Journal of organic chemistry, 78(7), 3421-3424 (2013-03-20)
A practical and safe synthesis of 1,2-bis(trimethylsilyl)benzene from 1,2-dichlorobenzene and Me3SiCl was achieved by use of a hybrid metal of Mg and CuCl in the presence of LiCl in 1,3-dimethyl-2-imidazolidinone (DMI). This method does not require a toxic HMPA, provides
Li-Ming Zhao et al.
Organic letters, 14(3), 886-889 (2012-01-24)
A highly α-regioselective prenylation of imines has been successfully developed. The efficiency of this approach is confirmed by a wide range of imines including N- and C-aryl aldimines, N-alkyl aldimines, C-alkyl aldimines, and N- and C-aryl ketimines. The approach uses
K Krüger et al.
Scandinavian journal of medicine & science in sports, 25(3), e283-e291 (2014-09-30)
Different types of exercise are characterized by the ability to induce specific physiological stimuli that might be able to induce the mobilization of progenitor cells. The aim of the current study was to investigate the mobilization of hematopoietic progenitor cells
Yolanda T Chong et al.
Cell, 161(6), 1413-1424 (2015-06-06)
Proteomics has proved invaluable in generating large-scale quantitative data; however, the development of systems approaches for examining the proteome in vivo has lagged behind. To evaluate protein abundance and localization on a proteome scale, we exploited the yeast GFP-fusion collection
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service