191523
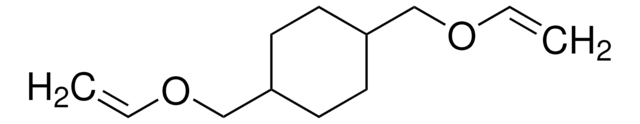
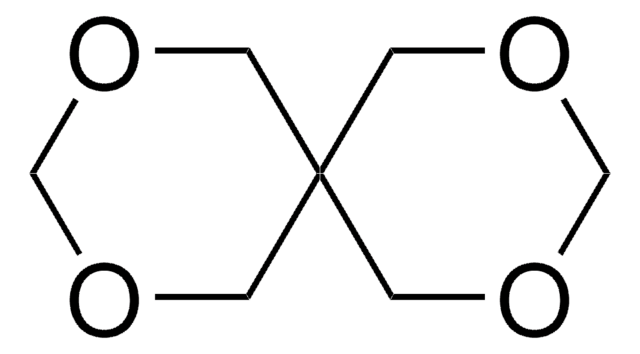
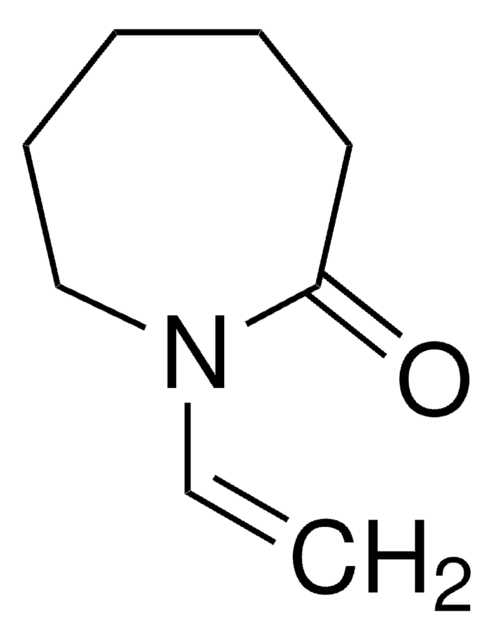
3,9-Divinyl-2,4,8,10-tetraoxaspiro[5.5]undecane
98%
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C11H16O4
CAS Number:
Molecular Weight:
212.24
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
bp
108-110 °C/2 mmHg (lit.)
mp
43-46 °C (lit.)
density
1.251 g/mL at 25 °C (lit.)
SMILES string
C=CC1OCC2(CO1)COC(OC2)C=C
InChI
1S/C11H16O4/c1-3-9-12-5-11(6-13-9)7-14-10(4-2)15-8-11/h3-4,9-10H,1-2,5-8H2
InChI key
OOXMQACSWCZQLX-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
3,9-Divinyl-2,4,8,10-tetraoxaspiro[5.5]undecane is an acetal-type crosslinking agent comonomer and has been used:
- in radical emulsion copolymerization of 2-hydroxyethyl methacrylate
- as cross-linking agent in the synthesis of acid-degradable core-crosslinked micelles
- in the synthesis of new biocompatible copolymer for loading the indomethacin as drug model
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Flash Point(F)
235.4 °F - closed cup
Flash Point(C)
113 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
S Stern et al.
Radiotherapy and oncology : journal of the European Society for Therapeutic Radiology and Oncology, 41(2), 143-149 (1996-11-01)
Solid tumours contain hypoxic cells which are resistant to radiotherapy. This study compares the efficacy of several strategies to counteract diffusion-limited hypoxia, or intermittent hypoxia in a fractionated regimen of 1 to 6 x 2 Gy. Nicotinamide (250 mg/kg), perflubron
Upon the emulsion polymerization of 2-hydroxyethyl methacrylate with 3, 9-divinyl-2, 4, 8, 10-tetraoxaspiro [5.5]-undecane.
Nita LE, et al.
Colloids and Surfaces. A, Physicochemical and Engineering Aspects, 381(1), 111-117 (2011)
Loredana E Nita et al.
Journal of materials science. Materials in medicine, 23(5), 1211-1223 (2012-03-15)
The study presents the possibility to use the 2-hydroxyethyl methacrylate--HEMA copolymer with a comonomer with spiroacetal moiety, 3,9-divinyl-2,4,8,10-tetraoxaspiro[5.5]-undecane)-U, as polymer network for loading the indomethacin--INN as drug model, and also, the controlled release evaluation of the prepared bioactive system. The
Acid-Degradable Core-Crosslinked Micelles Prepared from Thermosensitive Glycopolymers Synthesized via RAFT Polymerization.
Zhang L, et al.
Macromolecular Rapid Communications, 29(2), 123-129 (2008)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service