All Photos(2)
About This Item
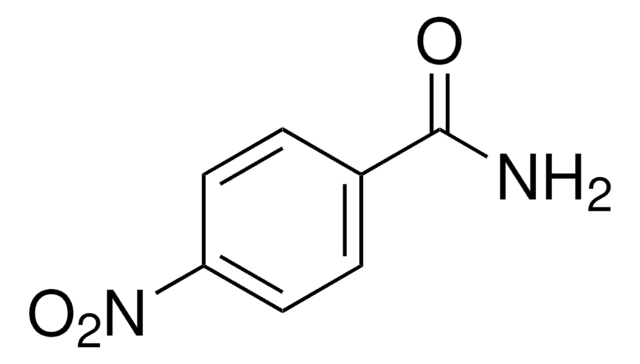
Linear Formula:
O2NC6H4CONH2
CAS Number:
Molecular Weight:
166.13
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
form
solid
mp
140-143 °C (lit.)
functional group
amide
SMILES string
NC(=O)c1cccc(c1)[N+]([O-])=O
InChI
1S/C7H6N2O3/c8-7(10)5-2-1-3-6(4-5)9(11)12/h1-4H,(H2,8,10)
InChI key
KWAYEPXDGHYGRW-UHFFFAOYSA-N
Related Categories
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Y Uchigata et al.
The Journal of biological chemistry, 257(11), 6084-6088 (1982-06-10)
We have shown previously that alloxan and streptozotocin, two major diabetogenic agents, cause DNA strand breaks in rat pancreatic islets and stimulate nuclear poly(ADP-ribose) synthetase, thereby depleting intracellular NAD level and inhibiting proinsulin synthesis (Okamoto, H. (1981) Mol. Cell. Biochem.
M R Purnell et al.
The Biochemical journal, 185(3), 775-777 (1980-03-01)
In a search for new inhibitors of the nuclear enzyme poly(ADP-ribose) synthetase, it was found that various benzamides substituted in the 3-position were the most inhibitory compounds found to date. Two of the benzamides, 3-aminobenzamide and 3-methoxybenzamide, were found to
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service