18805
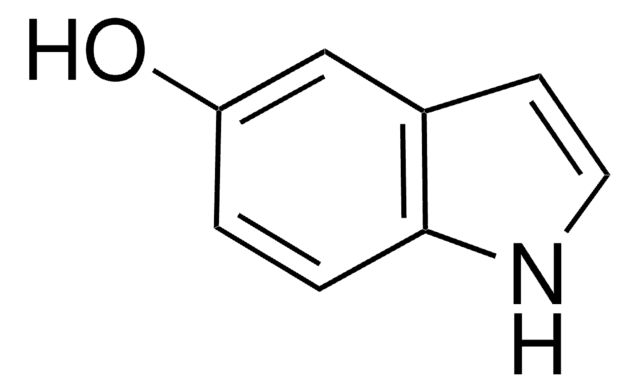
6-Hydroxyindole
≥99.0% (GC)
Synonym(s):
6-Indolol
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C8H7NO
CAS Number:
Molecular Weight:
133.15
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
≥99.0% (GC)
form
solid
mp
126-132 °C
SMILES string
Oc1ccc2cc[nH]c2c1
InChI
1S/C8H7NO/c10-7-2-1-6-3-4-9-8(6)5-7/h1-5,9-10H
InChI key
XAWPKHNOFIWWNZ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
- Reactant for preparation of tryptophan dioxygenase inhibitors pyridyl-ethenyl-indoles as potential anticancer immunomodulators
- Reactant for asymmetrical synthesis of notoamide J as a potential biosynthetic precursor of prenylated indole alkaloids
- Reactant for preparation of (quinolinyloxymethyl)isoxazolecarboxylate esters antituberculosis agents
- Reactant for preparation of indolyl(propanolamine) derivatives as HIV inhibitors
- Reactant for preparation of indoleoxyacetic acid derivatives as peroxisome proliferator-activated receptor agonists
- Reactant for preparation of 1-aroylindole 3-aroylindoles combretastatin A-4 analogs as antitumor agents and tubulin polymerization inhibitors
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Aquatic Chronic 2 - Eye Dam. 1 - Skin Sens. 1
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Jintae Lee et al.
Applied and environmental microbiology, 73(13), 4100-4109 (2007-05-08)
Since indole is present at up to 500 microM in the stationary phase and is an interspecies biofilm signal (J. Lee, A. Jayaraman, and T. K. Wood, BMC Microbiol. 7:42, 2007), we investigated hydroxyindoles as biofilm signals and found them
Damon Borg et al.
Journal of analytical toxicology, 41(1), 6-16 (2016-09-30)
Synthetic cannabinoids are a group of psychoactive compounds that mimic the effects of Δ9-tetrahydrocannabinol, the primary psychoactive constituent of marijuana (Cannabis sativa L). The Drug Enforcement Administration has classified many of the most common cannabinoids as Schedule 1 controlled substances.
Yoshimitsu Yamazaki et al.
Zeitschrift fur Naturforschung. C, Journal of biosciences, 65(1-2), 49-54 (2010-04-02)
A recent study showed that N-acylserotonin derivatives have strong inhibitory activity against tyrosinase. To clarify the role of the 5-hydroxy group in the indole ring, 2-, 4-, 5-, 6-, and 7-hydroxyindole and 11 related compounds such as 5-hydroxyindan and 6-hydroxyquinoline
Martin Švidrnoch et al.
Talanta, 150, 568-576 (2016-02-04)
Perfluoroheptanoic acid was employed as a volatile micellar phase in background electrolyte for micellar electrokinetic chromatography-tandem mass spectrometry separation and determination of 15 selected naphthoyl- and phenylacetylindole- synthetic cannabinoids and main metabolites derived from JWH-018, JWH-019, JWH-073, JWH-200 and JWH-250.
Stefan W Toennes et al.
Drug testing and analysis, 10(4), 644-650 (2017-10-03)
Each year, synthetic cannabinoids occur in high numbers on the illicit drug market, but data on their detectability are rarely available. A pilot study was performed to assess adverse effects of JWH-018, which is one of the oldest and best
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service