All Photos(1)
About This Item
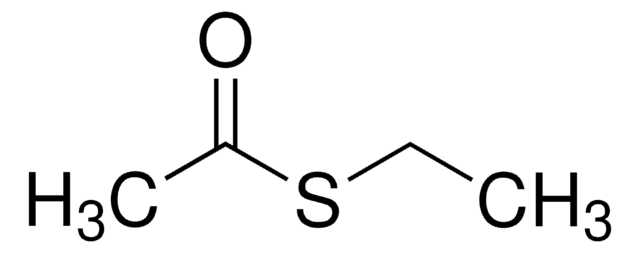
Linear Formula:
CH3COSC6H5
CAS Number:
Molecular Weight:
152.21
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
form
liquid
refractive index
n20/D 1.57 (lit.)
bp
99-100 °C/6 mmHg (lit.)
density
1.124 g/mL at 25 °C (lit.)
storage temp.
2-8°C
SMILES string
CC(=O)Sc1ccccc1
InChI
1S/C8H8OS/c1-7(9)10-8-5-3-2-4-6-8/h2-6H,1H3
InChI key
WBISVCLTLBMTDS-UHFFFAOYSA-N
Related Categories
Application
S-Phenyl thioacetate was used as a substrate to measure the esterase activity.
Storage Class Code
10 - Combustible liquids
WGK
WGK 2
Flash Point(F)
174.2 °F - closed cup
Flash Point(C)
79 °C - closed cup
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Yu Yuan et al.
Journal of the American Chemical Society, 131(15), 5432-5437 (2009-04-22)
Described herein is the chemical synthesis of the Cys(29)-Gly(77) glycopeptide domain (22) of erythropoietin. Our initial ligation strategy targeted a C --> N termini condensation between glycopeptide 3 and peptide 4. However, the reaction was hindered by the "unattainable" reactivity
K Lorentz et al.
Clinica chimica acta; international journal of clinical chemistry, 308(1-2), 69-78 (2001-06-20)
Arylesterase (EC 3.1.1.2) activity in serum was specifically measured using thiophenyl acetate in a mechanized assay at 37 degrees C with 4-bromophenylboronic acid as inhibitor of cholinesterase and hexacyanoferrate-III as indicator. The systematic development of a routine method, apparent limitations
Anita Bosak et al.
Molecules (Basel, Switzerland), 25(1) (2020-01-18)
Mammalian paraoxonase-1 hydrolyses a very broad spectrum of esters such as certain drugs and xenobiotics. The aim of this study was to determine whether carbamates influence the activity of recombinant PON1 (rePON1). Carbamates were selected having a variety of applications:
D I Draganov et al.
The Journal of biological chemistry, 275(43), 33435-33442 (2000-08-10)
The paraoxonase gene family contains at least three members: PON1, PON2, and PON3. The physiological roles of the corresponding gene products are still uncertain. Until recently, only the serum paraoxonase/arylesterase (PON1) had been purified and characterized. Here we report the
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service