179000
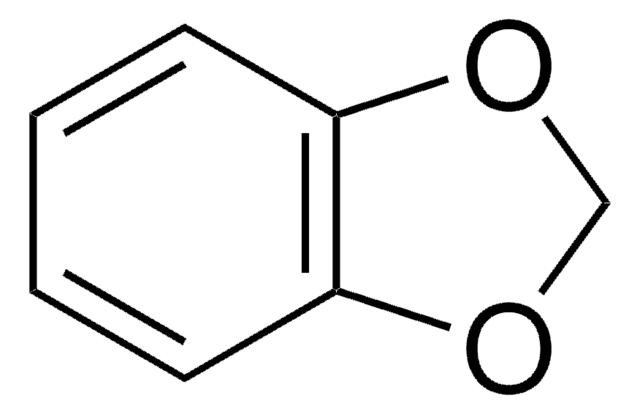
Benzo-1,4-dioxane
97%
Synonym(s):
1,2-Ethylenedioxybenzene, 1,4-Benzodioxan, 2,3-Dihydro-1,4-benzodioxin
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C8H8O2
CAS Number:
Molecular Weight:
136.15
Beilstein:
120846
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.21
Recommended Products
Quality Level
Assay
97%
form
liquid
refractive index
n20/D 1.549 (lit.)
bp
103 °C/6 mmHg (lit.)
density
1.142 g/mL at 25 °C (lit.)
SMILES string
C1COc2ccccc2O1
InChI
1S/C8H8O2/c1-2-4-8-7(3-1)9-5-6-10-8/h1-4H,5-6H2
InChI key
BNBQRQQYDMDJAH-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
The anti-inflammamtory properties and synthesis of carboxylic acid compounds containing benzo-1,4-dioxane (2,3-dihydro-1,4-benzodioxin) subunit was studied.
Application
Benzo-1,4-dioxane (2,3-dihydro-1,4-benzodioxin) was used in the synthesis of stereoisomers which were evaluated as α- and β- adrenergic antagonists.
Hazard Statements
Precautionary Statements
Hazard Classifications
Aquatic Chronic 3
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
156.2 °F - closed cup
Flash Point(C)
69 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
M T Vázquez et al.
Farmaco (Societa chimica italiana : 1989), 51(3), 215-217 (1996-03-01)
Synthesis and antiinflammatory properties of new carboxylic acids containing the 2,3-dihydro-1,4-benzodioxin subunit are described. The 2-(2,3-dihydro-1, 4-benzodioxin-6-yl)acetic acid was of comparable potency to Ibuprofen, in carrageenan induced rat paw edema assay.
M Khouili et al.
Farmaco (Societa chimica italiana : 1989), 51(3), 185-188 (1996-03-01)
The four stereoisomers of compound 1 were synthesized from 2,3-dihydro-1, 4-benzodioxin and evaluated as alpha- and beta-adrenergic antagonists. Enantiomer 1-b [2R, 2'S] (Figure 1) is the best beta 1-blocking agent. Furthermore all compounds showed a alpha-blocking activity.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service