177490
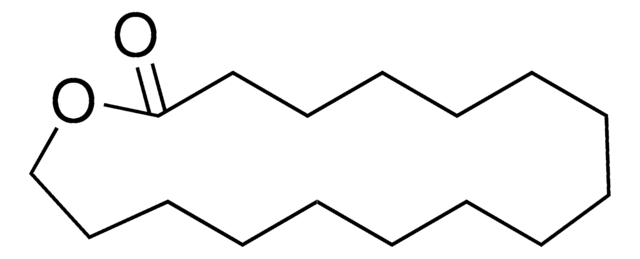
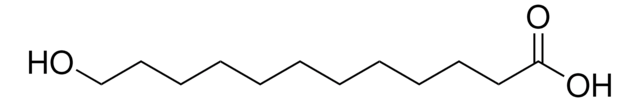
16-Hydroxyhexadecanoic acid
98%
Synonym(s):
16-Hydroxypalmitic acid, Juniperic acid
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
HO(CH2)15CO2H
CAS Number:
Molecular Weight:
272.42
Beilstein:
1783998
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
form
solid
mp
94-98 °C (lit.)
SMILES string
OCCCCCCCCCCCCCCCC(O)=O
InChI
1S/C16H32O3/c17-15-13-11-9-7-5-3-1-2-4-6-8-10-12-14-16(18)19/h17H,1-15H2,(H,18,19)
InChI key
UGAGPNKCDRTDHP-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
16-Hydroxyhexadecanoic acid was used in the synthesis of dihydroxypalmitic acids. It was also used to induce the expression of two GRP genes of Arabidopsis thaliana, AtGRP5 and AtGRP23.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Sapa Hima Rani et al.
The Journal of biological chemistry, 285(49), 38337-38347 (2010-10-06)
A key step in the triacylglycerol (TAG) biosynthetic pathway is the final acylation of diacylglycerol (DAG) by DAG acyltransferase. In silico analysis has revealed that the DCR (defective in cuticular ridges) (At5g23940) gene has a typical HX(4)D acyltransferase motif at
Akihisa Abe et al.
Journal of biochemistry, molecular biology, and biophysics : JBMBB : the official journal of the Federation of Asian and Oceanian Biochemists and Molecular Biologists (FAOBMB), 6(1), 37-43 (2002-08-21)
We have found that omega-hydroxy palmitic acid (16-hydroxy palmitic acid, omega-HPA) has both cell growth inhibiting and cell death inducing actions on human lung adenosquamous carcinoma cell line H596 and adenocarcinoma cell line A549. Further, these effects were dose- and
Jong Ho Park et al.
Plant physiology and biochemistry : PPB, 46(11), 1015-1018 (2008-07-29)
Glycine-rich proteins (GRPs) belong to a large family of heterogenous proteins that are enriched in glycine residues. The expression of two GRP genes of Arabidopsis thaliana, AtGRP5 and AtGRP23, was induced by 16-hydroxypalmitic acid (HPA), a major component of cutin.
G D Rees et al.
Biochimica et biophysica acta, 1257(3), 239-248 (1995-08-03)
Five microbial lipases from Chromobacterium viscosum, Candida cylindracea, Pseudomonas (source Fluka), Pseudomonas (source Genzyme) and lipoprotein lipase ex Microbial (Genzyme) have been screened for lactonisation activity towards 16-hydroxyhexadecanoic acid (HHA) in a variety of different w/o microemulsion systems. With the
Jean-Paul Douliez
Journal of colloid and interface science, 271(2), 507-510 (2004-02-20)
The interaction between cutin and suberin monomers, i.e., omega -hydroxylpalmitic acid, alpha, omega -hexadecanedioic acid, alpha, omega --hexadecanediol, 12-hydroxylstearic acid, and phospholipid vesicles biomimicking the lipid structure of plant cell membranes has been studied by optical and transmission electron microscopy
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service