All Photos(2)
About This Item
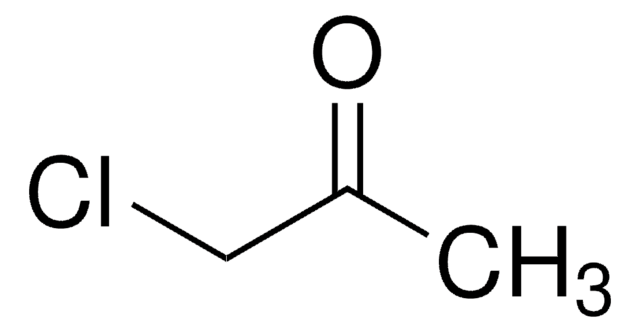
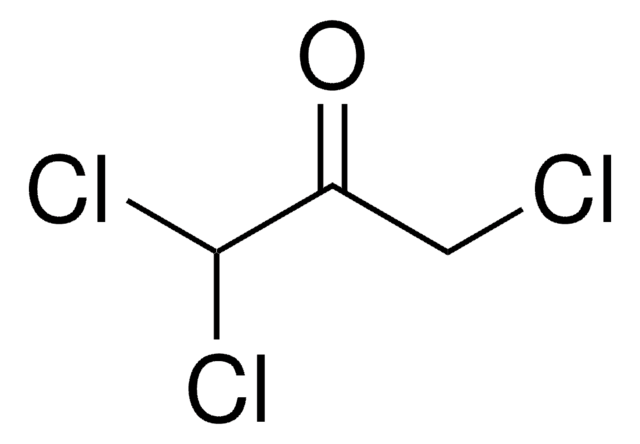
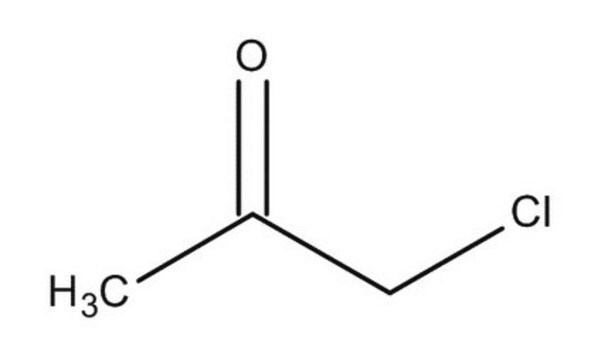
Linear Formula:
ClCH2COCH2Cl
CAS Number:
Molecular Weight:
126.97
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
vapor density
4.38 (vs air)
Quality Level
vapor pressure
<0.1 mmHg ( 20 °C)
Assay
≥95%
form
solid
bp
173 °C (lit.)
mp
39-41 °C (lit.)
solubility
alcohol: very soluble
diethyl ether: very soluble
water: soluble
density
1.383 g/mL at 25 °C (lit.)
storage temp.
2-8°C
SMILES string
ClCC(=O)CCl
InChI
1S/C3H4Cl2O/c4-1-3(6)2-5/h1-2H2
InChI key
SUNMBRGCANLOEG-UHFFFAOYSA-N
General description
1,3-Dichloroacetone has been identified as a metabolite of 1,3-dichloropropanol by TLC. Kinetics of the reversible hydration of 1,3-dichloroacetone have been studied spectrophotometrically in solutions of water in dioxan and in acetonitrile.
1,3-dichloroacetone is a dihaloketone derivative used as a reagent in the synthesis of complex multicyclic peptides.
1,3-dichloroacetone is a dihaloketone derivative used as a reagent in the synthesis of complex multicyclic peptides.
Application
1,3-Dichloroacetone was used as acceptor substrate in the cross-aldol reaction with donor substrates such as acetone, cyclopentanone and cyclohexanone.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 1 Inhalation - Acute Tox. 2 Dermal - Acute Tox. 2 Oral - Muta. 2 - Skin Corr. 1B
Storage Class Code
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Kinetics of the reversible hydration of 1, 3-dichloroacetone in dioxan and acetonitrile solution.
Bell RP, et al.
Proc. Royal Soc. Lond. B., 303(1472), 1-16 (1968)
Exploring the Use of Helicogenic Amino Acids for Optimising Single Chain Relaxin-3 Peptide Agonists.
Han Siean Lee et al.
Biomedicines, 8(10) (2020-10-18)
Relaxin-3 is a highly conserved two-chain neuropeptide that acts through its endogenous receptor the Relaxin Family Peptide-3 (RXFP3) receptor. The ligand/receptor system is known to modulate several physiological processes, with changes in food intake and anxiety-levels the most well studied
Ling Lu et al.
The Journal of organic chemistry, 64(3), 843-853 (2001-10-25)
Cross-aldol reactions of carbonyl compounds were achieved by the catalysis of SmI(2) or SmI(3), together with molecular sieves, at ambient temperature. 1,3-Dichloroacetone and 1-chloroacetone can be used as acceptor substrates in the cross-aldol reactions with donor substrates such as acetone
M Wojewodzka et al.
Chemico-biological interactions, 74(3), 221-231 (1990-01-01)
A number of polyamine (PA) derivatives of thiosemicarbazone of 1,3-dichloroacetone (TDA) have been prepared and their effect on growth in vivo of tumorigenic but not metastatic cell strain (LY-R) of mouse lymphoma L5178Y has been investigated. Polyamine derivatives of TDA
F B Daniel et al.
Drug and chemical toxicology, 16(4), 341-350 (1993-01-01)
1,3-Dichloropropanone (1,3-DCP) has been identified as a by-product of the chlorination of water and thus a potential contaminant in drinking water. Since little was known of its oral toxicity, subchronic exposure studies were conducted with male and female Sprague-Dawley rats
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service