All Photos(1)
About This Item
Linear Formula:
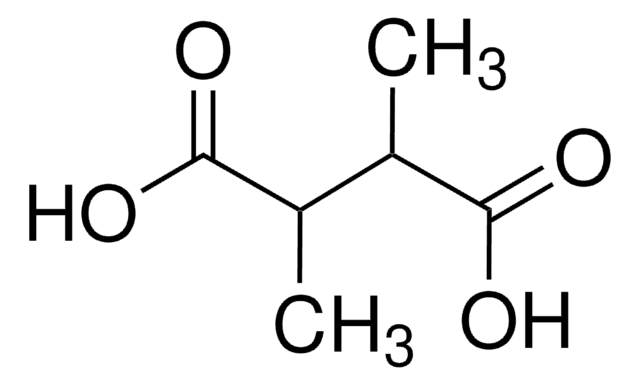
HO2CCH(CH3)CH(CH3)CO2H
CAS Number:
Molecular Weight:
146.14
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
99%
form
powder
mp
200 °C (dec.) (lit.)
SMILES string
C[C@@H]([C@@H](C)C(O)=O)C(O)=O
InChI
1S/C6H10O4/c1-3(5(7)8)4(2)6(9)10/h3-4H,1-2H3,(H,7,8)(H,9,10)/t3-,4+
InChI key
KLZYRCVPDWTZLH-ZXZARUISSA-N
Application
meso-2,3-Dimethylsuccinic acid was used in the synthesis of:
- meso-1,4-diiodo-2,3-dimethylbutane
- selenoacetal with an erythro-configured CHMe-CHMe fragment
- erythro-4-acetoxy-2,3-dimethylbutan-1-ol
- (±)-faranal
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Synthesis of (3S*, 4R*, 6E, 10Z)-3, 4, 7, 11-tetramethyltrideca-6, 10-dienal (faranal) using stereospecific 1, 4-cis-hydrogenation of conjugated double bonds.
Vasil'ev AA, et al.
Journal of the Chemical Society. Perkin Transactions 1, 14, 2211-2216 (2000)
Highly selective formation of eight-membered-ring systems by oxidative cyclization with molybdenum pentachloride-an environmentally friendly and inexpensive access to 2,2'-cyclolignans.
Beate Kramer et al.
Angewandte Chemie (International ed. in English), 41(16), 2981-2982 (2002-08-31)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service