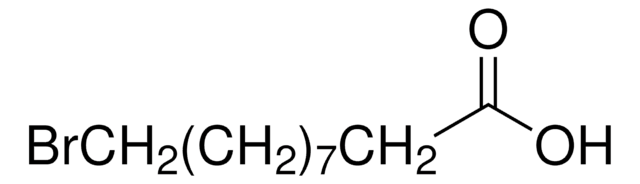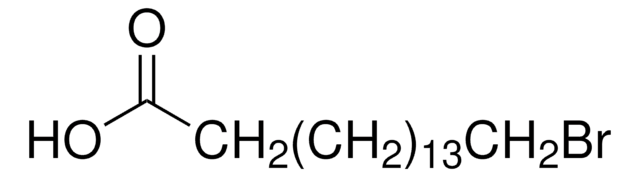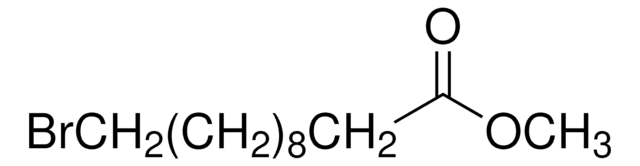All Photos(3)
About This Item
Linear Formula:
Br(CH2)10COOH
CAS Number:
Molecular Weight:
265.19
Beilstein:
1767205
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
grade
technical grade
Assay
95%
bp
173-174 °C/2 mmHg (lit.)
mp
45-48 °C (lit.)
SMILES string
OC(=O)CCCCCCCCCCBr
InChI
1S/C11H21BrO2/c12-10-8-6-4-2-1-3-5-7-9-11(13)14/h1-10H2,(H,13,14)
InChI key
IUDGNRWYNOEIKF-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
11-Bromoundecanoic acid reacts with potassium salt of dimethyl hydantoin to yield 4,4-dimethyl hydantoin-undecanoic acid.
Application
11-Bromoundecanoic acid was used in the synthesis of 11-phenoxyundecyl phosphate and 11-hydroxytetradecanoic acid.
Storage Class Code
11 - Combustible Solids
WGK
WGK 1
Flash Point(F)
235.4 °F - closed cup
Flash Point(C)
113 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Revathi V Padmanabhuni et al.
Industrial & engineering chemistry research, 51(14), 5148-5156 (2012-09-04)
The purpose of this study was to demonstrate that the surface of CaCO(3) fillers could be coated with an N-halamine based fatty acid to make the filler surface organophilic and accomplish antibacterial activity simultaneously, rendering the resulting polymer-filler composites antimicrobial.
I Navarro et al.
Bioorganic & medicinal chemistry, 4(3), 439-443 (1996-03-01)
The synthesis of deuterium labeled 11- and 12-hydroxytetradecanoic acids to study a (11E) desaturase in the moth Spodoptera littoralis is reported. [14,14,14-2H3] 12-hydroxytetradecanoic acid was synthesized in four steps from 11-iodo-1-undecene in 49% overall yield. Deuterium was introduced by reaction
L L Danilov et al.
Bioorganicheskaia khimiia, 35(3), 431-432 (2009-07-22)
A new scheme of synthesis of 11-phenoxyundecyl phosphate from 11-bromoundecanoic acid was suggested for its ability to react as an acceptor of 2-acetamido-2-deoxy-alpha-D-glucopyranosyl phosphate in a reaction catalyzed by UDP-N-acetylglucosamine : polyprenyl phosphate N-acetylglucosamine phosphotransferase from Salmonella arizona O:59.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service