163457
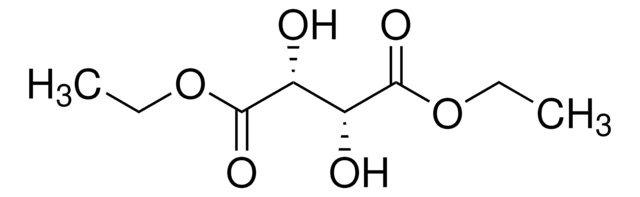
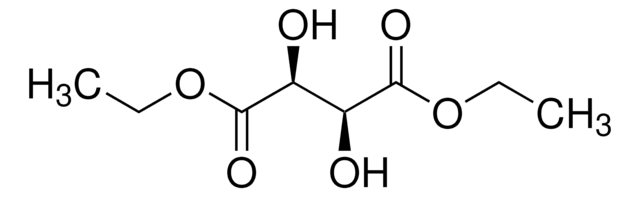
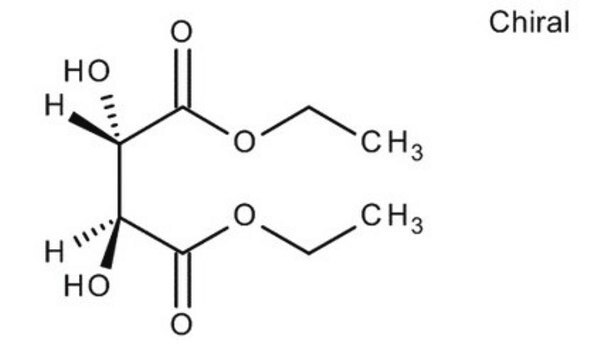
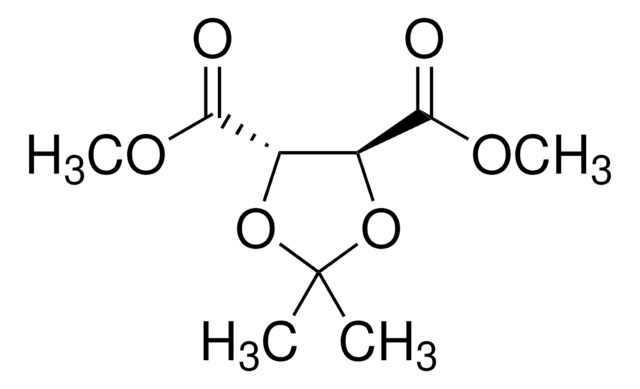
(+)-Dimethyl L-tartrate
99%
Synonym(s):
L-(+)-Tartaric acid dimethyl ester
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
[-CH(OH)CO2CH3]2
CAS Number:
Molecular Weight:
178.14
Beilstein:
1726256
EC Number:
MDL number:
UNSPSC Code:
12352108
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
99%
form
solid
optical activity
[α]22/D +21°, c = 2.5 in H2O
optical purity
ee: ≥99% (GLC)
bp
163 °C/23 mmHg (lit.)
mp
57-60 °C (dec.) (lit.)
density
1.238 g/mL at 25 °C (lit.)
SMILES string
COC(=O)[C@H](O)[C@@H](O)C(=O)OC
InChI
1S/C6H10O6/c1-11-5(9)3(7)4(8)6(10)12-2/h3-4,7-8H,1-2H3/t3-,4-/m1/s1
InChI key
PVRATXCXJDHJJN-QWWZWVQMSA-N
Looking for similar products? Visit Product Comparison Guide
Application
(+)-Dimethyl L-tartrate can react with sulfur tetrafluoride to form dimethyl meso-2,3-difluorosuccinate.
It may be used in the synthesis of the following chiral ligands for use in asymmetric synthesis:
It may be used in the synthesis of the following chiral ligands for use in asymmetric synthesis:
- (2R, 3R)-1,4-dimethoxy-1,1,4,4-tetraphenyl-2,3-butanediol
- (-)-(4S,5R)-4-(2-pyridyl)-5-(diphenylphosphino)methyl-2,2-dimethyl-1,3-dioxolane (PYDIPHOS)
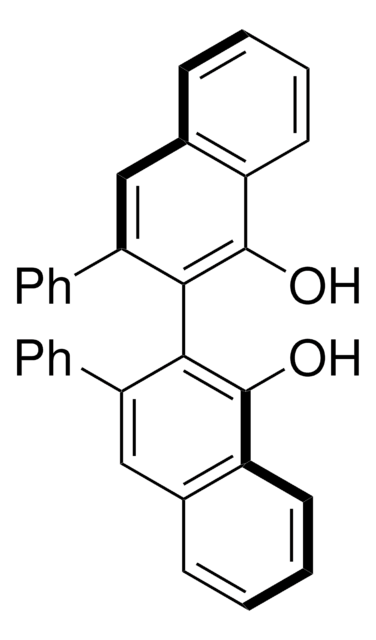
- (4R,5R)-α,α,α′,α′-2,2-hexaphenyl-4,5-dimethanol-1,3-dioxolane
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Synthesis and structure of (4R, 5R)-a, a, a', a'-2, 2-hexaphenyl-4, 5-dimethanol-1, 3-dioxolane.
Irurre J, et al.
Tetrahedron Asymmetry, 3(12), 1591-1596 (1992)
(-)-(4S, 5R)-4-(2-pyridyl)-5-(diphenylphosphino) methyl-2, 2-dimethyl-1, 3-dioxolane a new chiral ligand for enantioselective catalysis.
Chelucci G, et al.
Tetrahedron Asymmetry, 5(3), 299-302 (1994)
Synthesis of (2R, 3R)-1, 4-dimethoxy-1, 1, 4, 4-tetraphenyl-2, 3-butanediol: A new C2-symmetric vicinal diol from dimethyl L-tartrate.
Nakayama K and Rainier JD.
Tetrahedron, 46(12), 4165-4170 (1990)
Treatment of dimethyl (+)-L-tartrate with sulfur tetrafluoride.
Burmakov AI, et al.
Journal of Fluorine Chemistry, 19(2), 151-161 (1981)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service