159239
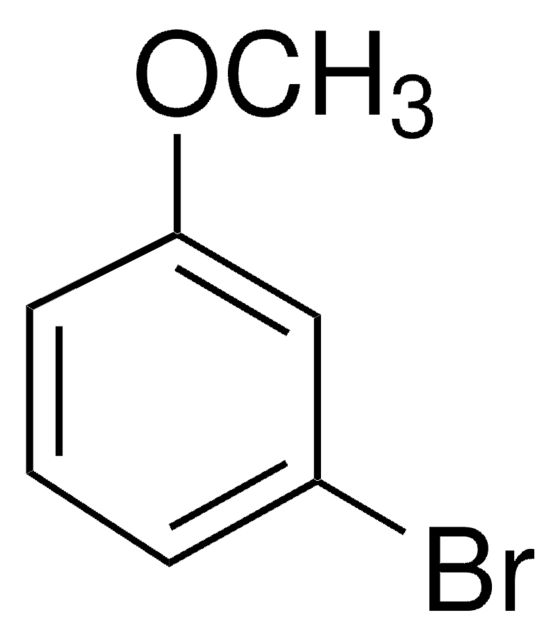
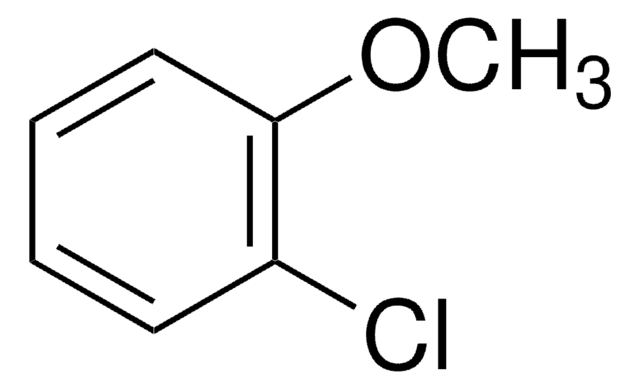
2-Bromoanisole
97%
Synonym(s):
1-Bromo-2-methoxybenzene
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
BrC6H4OCH3
CAS Number:
Molecular Weight:
187.03
Beilstein:
1859996
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
form
liquid
refractive index
n20/D 1.573 (lit.)
bp
223 °C (lit.)
mp
2 °C (lit.)
density
1.502 g/mL at 25 °C (lit.)
SMILES string
COc1ccccc1Br
InChI
1S/C7H7BrO/c1-9-7-5-3-2-4-6(7)8/h2-5H,1H3
InChI key
HTDQSWDEWGSAMN-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Diffusion coefficients of 2-bromoanisole at infinite dilution in supercritical carbon dioxide has been evaluated by Taylor-Aris chromatographic technique.
Application
2-Bromoanisole was used in the synthesis of unsymmetrically substituted biphenyl compounds. It was also used in the preparation of the family of exo-[n.m.n.m]metacyclophanes (n,m > or = 3).
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Aquatic Chronic 3 - Eye Irrit. 2 - Skin Irrit. 2
Storage Class Code
10 - Combustible liquids
WGK
WGK 1
Flash Point(F)
208.4 °F - closed cup
Flash Point(C)
98 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Patrick T Weiser et al.
Bioorganic & medicinal chemistry, 22(2), 917-926 (2013-12-24)
A series of unsymmetrically substituted biphenyl compounds was designed as alpha helical proteomimetics with the aim of inhibiting the binding of coactivator proteins to the nuclear hormone receptor coactivator binding domain. These compounds were synthesized in good overall yields in
Burns et al.
The Journal of organic chemistry, 65(17), 5185-5196 (2000-09-19)
A general strategy for the preparation of the family of exo-[n.m.n.m]metacyclophanes (n,m > or = 3) in 6-steps (starting from 2-bromoanisole) that utilizes a [2 + 2] approach to furnish the exo-metacyclophane ring in good to moderate yield is described.
Zhishan Xu et al.
Cancer letters, 447, 75-85 (2019-01-24)
Herein we present half-sandwich IrIII complexes [(η5-Cpxbiph)Ir(OˆC)Cl] containing OˆC(NHC)-chelating ligand as anticancer and antimetastasis agents. All the complexes displayed high potency in vitro against a wide range of cancer cells. In addition, Ir2 significantly curb tumor growth in a colon
Diffusion coefficients of 2-fluoroanisole, 2-bromoanisole, allylbenzene and 1, 3-divinylbenzene at infinite dilution in supercritical carbon dioxide
Suarez-Iglesias O, et al.
Fluid Phase Equilibria, 260(2), 279-286 (2007)
Nicholas C Pflug et al.
Environmental science & technology, 53(9), 4813-4822 (2019-03-27)
Anilines have been shown to be especially susceptible to single-electron oxidation by excited triplet-state photosensitizers (3sens*), and thus, are good potential candidates to probe the oxidative properties of triplet-state chromophoric dissolved organic matter (3CDOM*). However, steady-state experiments tend to underestimate
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service