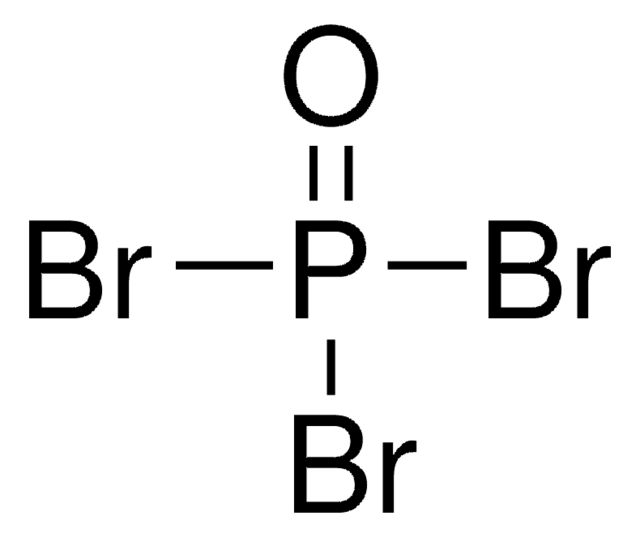157783
Phosphorus tribromide
97%
Synonym(s):
Phosphorus(III) bromide
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
PBr3
CAS Number:
Molecular Weight:
270.69
EC Number:
MDL number:
UNSPSC Code:
12352101
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
vapor pressure
0.27 psi ( 54 °C)
Quality Level
Assay
97%
form
liquid
refractive index
n20/D 1.697 (lit.)
bp
175 °C (lit.)
mp
−41.5 °C (lit.)
density
2.88 g/mL at 20 °C (lit.)
SMILES string
BrP(Br)Br
InChI
1S/Br3P/c1-4(2)3
InChI key
IPNPIHIZVLFAFP-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Phosphorus tribromide (PBr3) is commonly used in Hell-Volhard-Zelinsky halogenation for the a-bromination of carboxylic acids to form the corresponding acyl bromide. It is also useful for the conversion of primary and secondary alcohols to alkyl bromides.
Application
Phosphorus tribromide may be used as a reagent:
- In a novel protocol for synthesizing 7-ethoxy-1-(p-ethylphenoxy)-3,7-dimethyl-2-octene, a synthetic juvenile hormone mimic of trans,trans,cis-10,11-epoxy-7-ethyl-3,11-dimethyltrideca-2,6-dienoate.
- To synthesize 1,2,3-diazaphosphinane, 1,2,3-thiazaphosphinine and 1,2-azaphosphole bearing a chromone ring.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Dam. 1 - Skin Corr. 1B - STOT SE 3
Target Organs
Respiratory system
Supplementary Hazards
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Organic Chemistry, 1 (1988)
Organic Chemistry (2008)
Reaction of 2-cyano-3-(4-oxo-4H-chromen-3-yl) prop-2-enamide with some phosphorus reagents: synthesis of some novel diethyl phosphonates, 1, 2, 3-diazaphosphinanes, 1, 2, 3-thiazaphosphinine and 1, 2-azaphospholes bearing a chromone ring.
Ali TE & Hassan MM
Research on Chemical Intermediates, 44(1), 173-189 (2018)
Phosphorus tribromide promoted allylic rearrangement of a tertiary vinyl carbinol. Stereochemistry of the reaction product and application to the synthesis of JH-25, a potent juvenile hormone mimic.
Babler JH
The Journal of Organic Chemistry, 41(7), 1262-1264 (1976)
Andrey S Mereshchenko et al.
Nature chemistry, 7(7), 562-568 (2015-06-24)
'Roaming' is a new and unusual class of reaction mechanism that has recently been discovered in unimolecular dissociation reactions of isolated molecules in the gas phase. It is characterized by frustrated bond cleavage, after which the two incipient fragments 'roam'
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service