148083
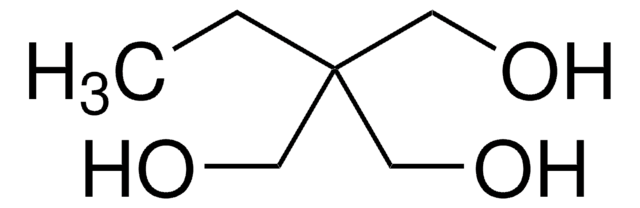
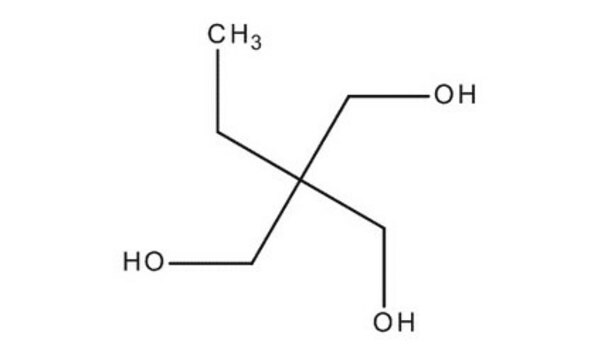
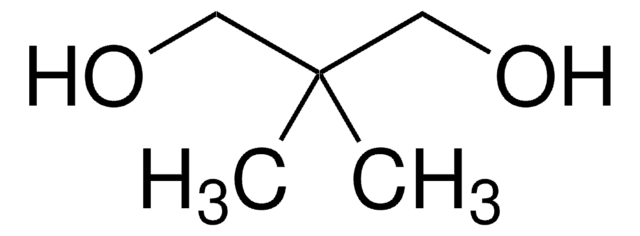
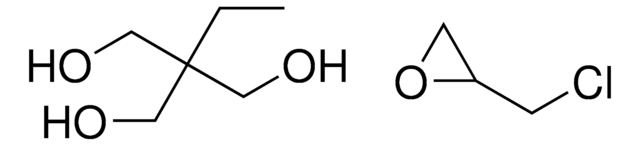
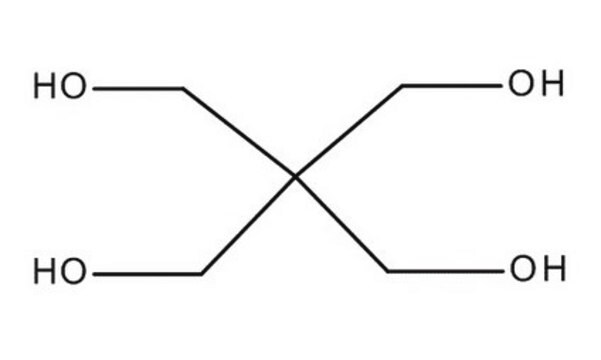
1,1,1-Tris(hydroxymethyl)propane
97%
Synonym(s):
2-Ethyl-2-hydroxymethyl-1,3-propanediol, Trimethylolpropane
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
CH3CH2C(CH2OH)3
CAS Number:
Molecular Weight:
134.17
Beilstein:
1698309
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
vapor density
4.8 (vs air)
Quality Level
vapor pressure
<1 mmHg ( 20 °C)
Assay
97%
form
(Powder or Crystals or Granules or Chunks)
autoignition temp.
1301 °F
bp
159-161 °C/2 mmHg (lit.)
mp
56-58 °C (lit.)
SMILES string
CCC(CO)(CO)CO
InChI
1S/C6H14O3/c1-2-6(3-7,4-8)5-9/h7-9H,2-5H2,1H3
InChI key
ZJCCRDAZUWHFQH-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
1,1,1-Tris(hydroxymethyl)propane was used as hydrogen bond-donating agent during triol-promoted activation of the C-F bond of benzylic fluorides. It was used in synthesis of new octanuclear manganese cluster and hyperbranched polyethers.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Repr. 2
Storage Class Code
11 - Combustible Solids
WGK
WGK 1
Flash Point(F)
356.0 °F - Cleveland open cup
Flash Point(C)
180 °C - Cleveland open cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Hyperbranched aliphatic polyethers obtained from environmentally benign monomer: glycerol carbonate.
Rokicki G, et al.
Green Chemistry, 7(7), 529-539 (2005)
Pier Alexandre Champagne et al.
Beilstein journal of organic chemistry, 9, 2451-2456 (2013-12-25)
Activation of the C-F bond of benzylic fluorides was achieved using 1,1,1-tris(hydroxymethyl)propane (2) as a hydrogen bond-donating agent. Investigations demonstrated that hydrogen bond-donating solvents are promoting the activation and hydrogen bond-accepting ones are hindering it. However, the reaction is best
Y Y Linko et al.
Journal of biotechnology, 66(1), 41-50 (1998-12-29)
The interest in the applications of biocatalysis in organic syntheses has rapidly increased. In this context, lipases have recently become one of the most studied groups of enzymes. We have demonstrated that lipases can be used as biocatalyst in the
Constantinos J Milios et al.
Dalton transactions (Cambridge, England : 2003), (2)(2), 351-356 (2005-12-21)
The reaction between MnBr(2).4H(2)O with H(3)tmp (1,1,1-tris(hydroxymethyl)propane) in MeCN in the presence of Na(O(2)CCMe(3)) and NBu(4)Br produces the complex [Mn(8)(O(2)CCMe(3))(2)(tmp)(2)(Htmp)(4)Br(4)(H(2)O)(2)].2MeCN (1.2MeCN) in good yield. The centrosymmetric octanuclear molecule consists of four Mn(III) and four Mn(II) ions assembled together by fourteen
Zhongyu Li et al.
Bioconjugate chemistry, 22(3), 518-522 (2011-02-11)
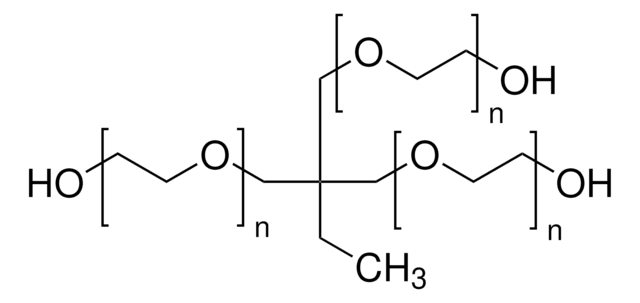
A synthetic route to prepare acetal-protected heterobifunctional poly(ethylene glycol), allyl(1-ethoxyethoxy)-PEG-OH (allyl(EE)-PEG-OH), was successfully established using a newly synthesized initiator, trimethylolpropane allyl (1-ethoxyethoxy) ether (TMPAEEE). Heterobifunctional allyl(OH)-mPEG and heterotrifunctional allyl(OH)-PEG-alkyne were obtained, respectively, after modification from this precursor polymer. The polymers
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service