All Photos(2)
About This Item
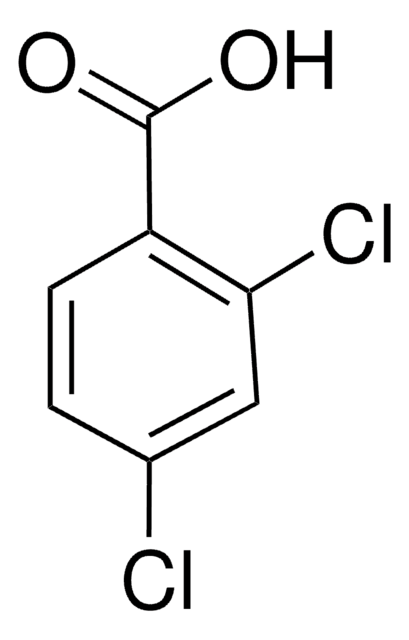
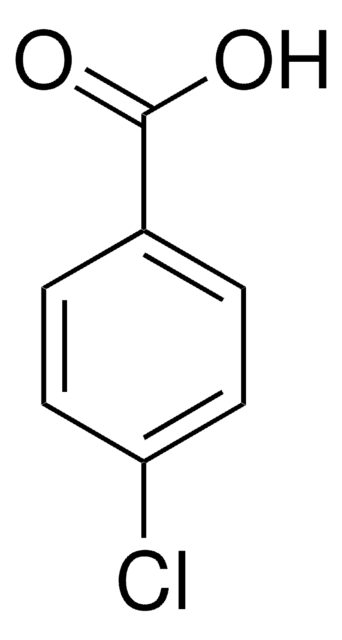
Linear Formula:
Cl2C6H3CO2H
CAS Number:
Molecular Weight:
191.01
Beilstein:
2044777
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
99%
form
solid
mp
204-206 °C (lit.)
SMILES string
OC(=O)c1ccc(Cl)c(Cl)c1
InChI
1S/C7H4Cl2O2/c8-5-2-1-4(7(10)11)3-6(5)9/h1-3H,(H,10,11)
InChI key
VPHHJAOJUJHJKD-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
3,4-Dichlorobenzoic acid was employed as internal standard during the multiresidue analysis of pharmaceuticals and personal care products by ultra performance liquid chromatography-positive/negative electrospray tandem mass spectrometry. It was used to study the metabolic fate of 4-chloro-3,5-dinitrobenzoic acid.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Fate of substituted benzoates in the freshwater green alga, Chlamydomonas reinhardtii 11-32b.
Gutenkauf A, et al.
Biodegradation, 9(5), 359-368 (1998)
Barbara Kasprzyk-Hordern et al.
Analytical and bioanalytical chemistry, 391(4), 1293-1308 (2008-02-07)
The main aim of the presented research is to introduce a new technique, ultra performance liquid chromatography-positive/negative electrospray tandem mass spectrometry (UPLC-ESI/MS/MS), for the development of new simultaneous multiresidue methods (over 50 compounds). These methods were used for the determination
K Umehara et al.
Drug metabolism and disposition: the biological fate of chemicals, 28(8), 887-894 (2000-07-20)
The metabolism of 1-(3,4-dichlorobenzyl)-5-octylbiguanide (OPB-2045), a new potent biguanide antiseptic, was investigated using rat and dog liver preparations to elucidate the mechanism of OPB-2045 metabolite formation, in which the octyl side chain is reduced to four, five, or six carbon
P Adriaens et al.
Applied and environmental microbiology, 57(1), 173-179 (1991-01-01)
When Acinetobacter sp. strain 4-CB1 was grown on 4-chlorobenzoate (4-CB), it cometabolized 3,4-dichlorobenzoate (3,4-DCB) to 3-chloro-4-hydroxybenzoate (3-C-4-OHB), which could be used as a growth substrate. No cometabolism of 3,4-DCB was observed when Acinetobacter sp. strain 4-CB1 was grown on benzoate.
Yoshiteru Noutoshi et al.
Scientific reports, 2, 705-705 (2012-10-11)
Plant activators are agrochemicals that protect crops from pathogens. They confer durable resistance to a broad range of diseases by activating intrinsic immune mechanisms in plants. To obtain leads regarding useful compounds, we have screened a chemical library using an
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service