143936
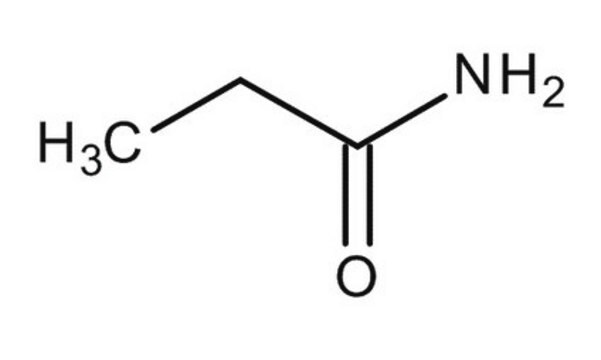
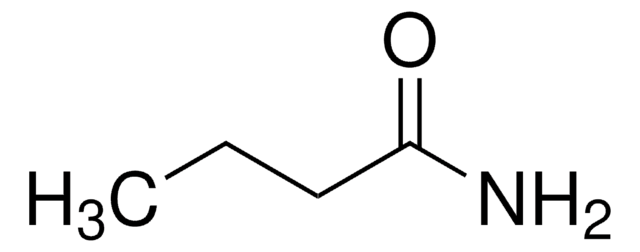
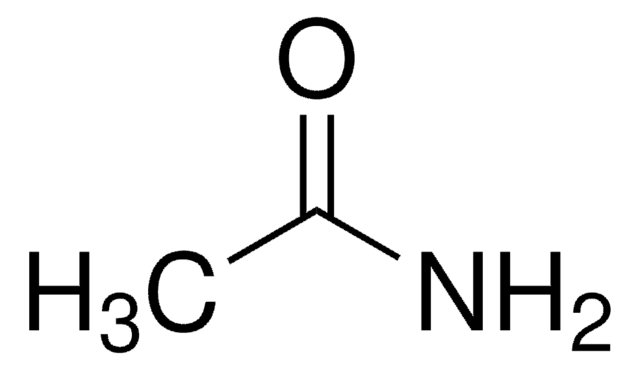
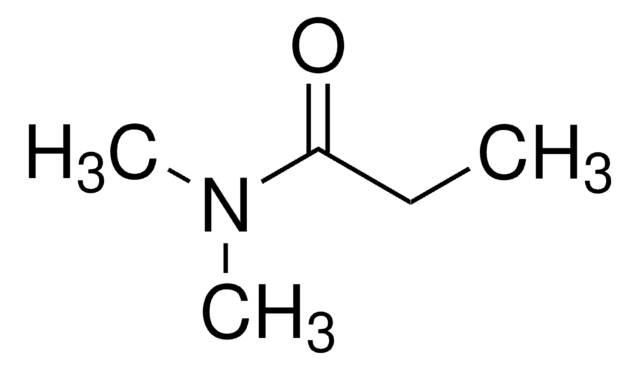
Propionamide
97%
Synonym(s):
Propanamide, Propylamide
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Linear Formula:
CH3CH2CONH2
CAS Number:
Molecular Weight:
73.09
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
bp
213 °C (lit.)
mp
76-79 °C (lit.)
solubility
alcohol: freely soluble
chloroform: freely soluble
diethyl ether: freely soluble
water: freely soluble
density
1.042 g/mL at 25 °C (lit.)
SMILES string
CCC(N)=O
InChI
1S/C3H7NO/c1-2-3(4)5/h2H2,1H3,(H2,4,5)
InChI key
QLNJFJADRCOGBJ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Propionamide on γ-irradiation reacts with sulfur dioxide and this reaction has been studied by ESR spectroscopy, gas absorption measurements and X-ray diffraction.
Application
Propionamide was used as adsorbent in the determination of adsorption isotherms of acetamide and propionamide on multi-wall carbon nanotube. It was used in a robust screening method to study biotransformations using (+)-γ-lactamase enzyme.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Reactions of Gases with Irradiated Organic Solids: III. Reactions of Propionamide, n-Butyramide, Isobutyramide, Methacrylamide, Valeramide, and Stearamide with Sulfur Dioxide.
Perotti A, et al.
Mol. Cryst. Liq. Cryst., 9(1), 323-342 (2969)
Ramesh Narayanan et al.
Molecular endocrinology (Baltimore, Md.), 22(11), 2448-2465 (2008-09-20)
Androgen receptor (AR) ligands are important for the development and function of several tissues and organs. However, the poor oral bioavailability, pharmacokinetic properties, and receptor cross-reactivity of testosterone, coupled with side effects, place limits on its clinical use. Selective AR
J D Stone et al.
Toxicology and applied pharmacology, 161(1), 50-58 (1999-11-24)
Neurofilament modification and accumulation, occurring in toxicant-induced neuropathies, has been proposed to compromise fast axonal transport and contribute to neurological symptoms or pathology. The current study compares the effects of the neurotoxicants acrylamide (ACR) and 2,5-hexanedione (2,5-HD) on the quantity
Study of Adsorption Isotherms of Acetamide and Propionamide on Carbon Nanotube.
Vadi M and Moradi N.
Orient. J. Chem., 27(4), 1491-1491 (2011)
Dale W Sickles et al.
Toxicology and applied pharmacology, 222(1), 111-121 (2007-06-02)
The microtubule (MT) motor protein kinesin is a vital component of cells and organs expressing acrylamide (ACR) toxicity. As a mechanism of its potential carcinogenicity, we determined whether kinesins involved in cell division are inhibited by ACR similar to neuronal
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service