143774
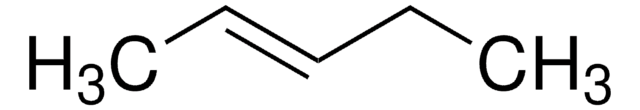
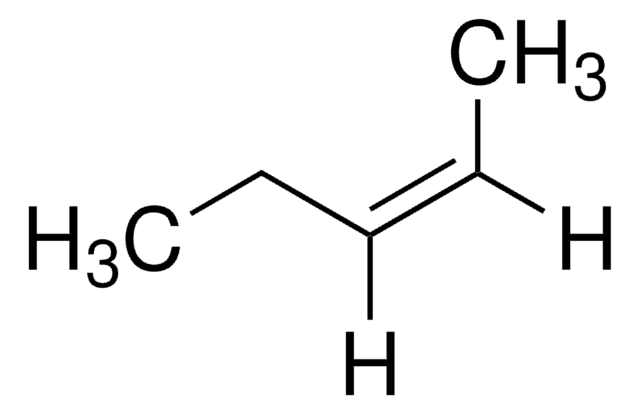
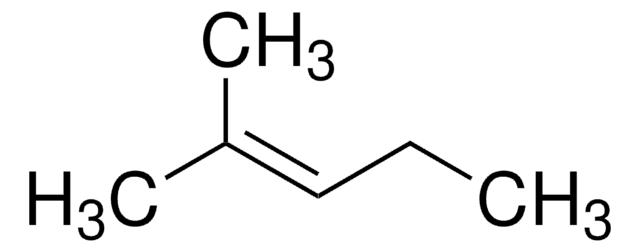
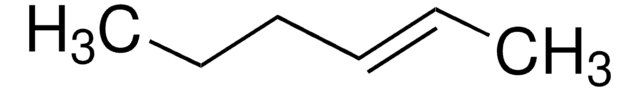
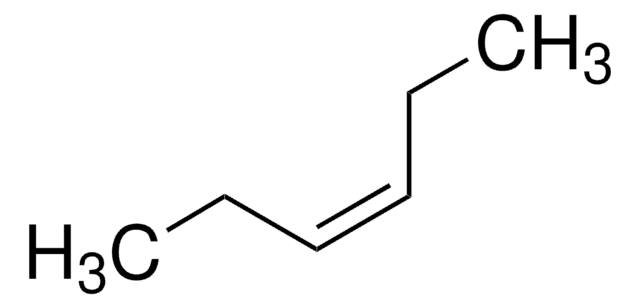
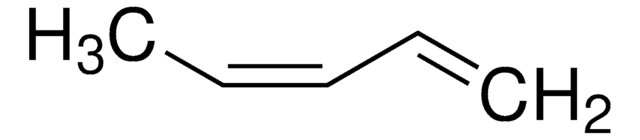
2-Pentene (mixture of cis and trans)
99%
Synonym(s):
β-Amylene, β-n-Amylene, 1-Methyl-2-ethylethylene, 3-Pentene, sym-Methylethylethylene
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
CH3CH2CH=CHCH3
CAS Number:
Molecular Weight:
70.13
Beilstein:
969151
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
vapor pressure
8.06 psi ( 20 °C)
Quality Level
Assay
99%
form
liquid
refractive index
n20/D 1.38 (lit.)
bp
37 °C (lit.)
density
0.65 g/mL at 25 °C (lit.)
storage temp.
2-8°C
SMILES string
CCC=CC
InChI
1S/C5H10/c1-3-5-4-2/h3,5H,4H2,1-2H3/b5-3+
InChI key
QMMOXUPEWRXHJS-HWKANZROSA-N
Related Categories
General description
2-Pentene undergoes addition reaction with phenoxathiin cation radical in acetonitrile solution to form bis(10-phenoxathiiniumyl)alkane adducts.
Application
2-Pentene (mixture of cis and trans) was used in the synthesis of polycyclopentene by ring-opening metathesis polymerization.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Asp. Tox. 1 - Flam. Liq. 2
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
-49.0 °F - closed cup
Flash Point(C)
-45 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Synthesis of narrow-distribution polycyclopentene using a ruthenium ring-opening metathesis initiator.
Myers SB and Register RA.
Polymer, 49(4), 877-882 (2008)
Bing-Jun Zhao et al.
The Journal of organic chemistry, 72(16), 6154-6161 (2007-07-03)
Addition of phenoxathiin cation radical (PO*+) to acyclic alkenes in acetonitrile (MeCN) solution occurred stereospecifically to form bis(10-phenoxathiiniumyl)alkane adducts. Stereospecific trans addition is ascribed to the intermediacy of an episulfonium cation radical. The alkenes used were cis- and trans-2-butene, cis-
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service