All Photos(1)
About This Item
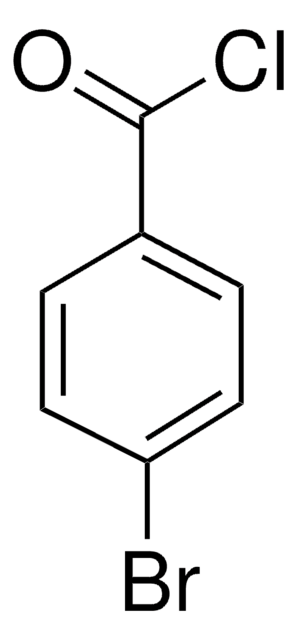
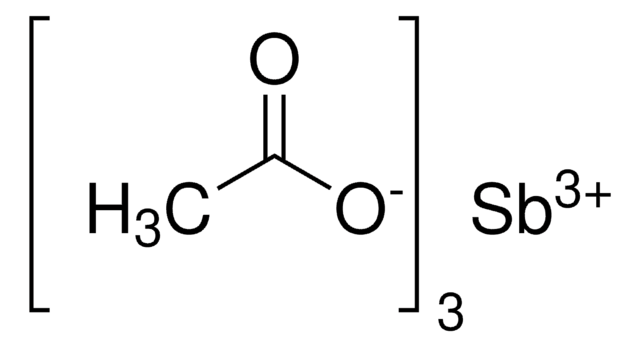
Linear Formula:
C6H5COBr
CAS Number:
Molecular Weight:
185.02
Beilstein:
1855439
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
refractive index
n20/D 1.589 (lit.)
bp
218-219 °C (lit.)
density
1.57 g/mL at 25 °C (lit.)
SMILES string
BrC(=O)c1ccccc1
InChI
1S/C7H5BrO/c8-7(9)6-4-2-1-3-5-6/h1-5H
InChI key
AQIHMSVIAGNIDM-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Benzoyl bromide is a versatile reagent which causes the benzoylation of ethers.
Application
Benzoyl bromide was used to study the kinetics of solvolysis of benzoyl halides in microemulsions of sodium bis(2-ethylhexyl)sulfosuccinate/isooctane/water at 25°C.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Skin Corr. 1B - STOT SE 3
Target Organs
Respiratory system
Supplementary Hazards
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
194.0 °F - closed cup
Flash Point(C)
90 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
L García-Río et al.
Langmuir : the ACS journal of surfaces and colloids, 22(18), 7499-7506 (2006-08-23)
A study was carried out on the solvolysis reactions of different benzoyl halides in microemulsions of water/NH4DEHP/isooctane, where NH4DEHP is ammonium bis(2-ethylhexyl) phosphate. Because of the low solubility of benzoyl halides in water, they are distributed between the continuous medium
Tülay Polat et al.
Carbohydrate research, 338(5), 447-449 (2003-02-01)
A simple and efficient method is developed for the chemoselective one-pot conversion of ethers (benzyl, TBDMS and acetal) to the corresponding benzoates by zinc triflate-catalyzed deprotection and benzoylation by benzoyl bromide. In the same reaction, methyl or p-methoxyphenyl glycosides are
Mai Do et al.
Nanoscale, 11(37), 17262-17269 (2019-06-28)
Perovskite nanoparticles have attracted the attention of research groups around the world for their impressive photophysical properties, facile synthesis and versatile surface chemistry. Here, we report a synthetic route that takes advantage of a suite of soluble precursors to generate
Dmitry Baranov et al.
ACS nano, 15(1), 650-664 (2020-12-23)
Excitonic coupling, electronic coupling, and cooperative interactions in self-assembled lead halide perovskite nanocrystals were reported to give rise to a red-shifted collective emission peak with accelerated dynamics. Here we report that similar spectroscopic features could appear as a result of
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service