132225
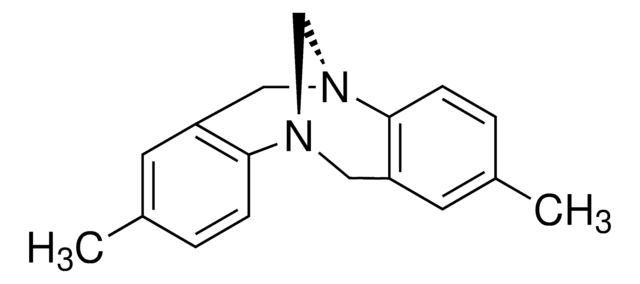
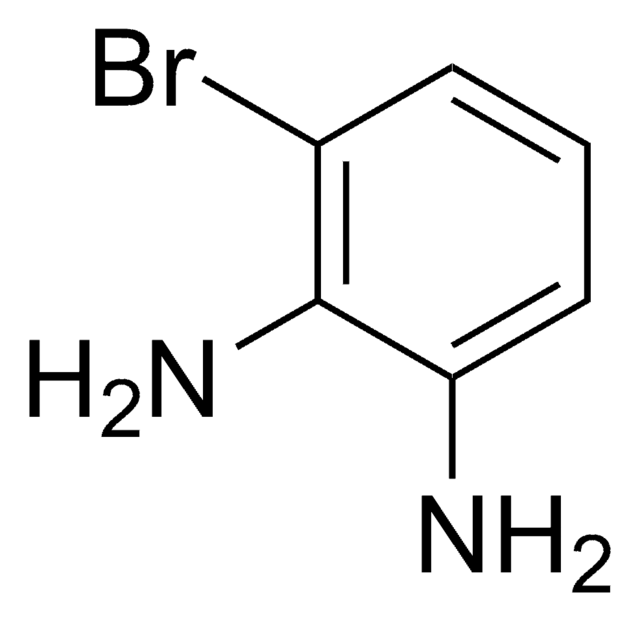
Troger′s Base
98%
Synonym(s):
2,8-Dimethyl-6H,12H-5,11-methanodibenzo[b,f][1,5]diazocine
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C17H18N2
CAS Number:
Molecular Weight:
250.34
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
form
powder
technique(s)
photometry: suitable
mp
133-136 °C (lit.)
SMILES string
Cc1ccc2[N@@H]3C[N@@H](Cc2c1)c4ccc(C)cc4C3
InChI
1S/C17H18N2/c1-12-3-5-16-14(7-12)9-18-11-19(16)10-15-8-13(2)4-6-17(15)18/h3-8H,9-11H2,1-2H3
InChI key
SXPSZIHEWFTLEQ-UHFFFAOYSA-N
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
R A Johnson et al.
Journal of medicinal chemistry, 36(21), 3202-3206 (1993-10-15)
The synthesis of 2,8-dimethyl-6H,12H-5,11-methanodibenzo[b,f][1,5]diazocine (Tröger's base) from p-toluidine and of two Tröger's base analogs from other anilines by reaction with hexamethylenetetramine in trifluoroacetic acid is described. 2,3,6,7-Tetrahydro-9-methyl-2,6-di-p-tolyl-1H,5H-pyrimido[5,6,1-ij] quinazoline is formed as a secondary product in the reaction of p-toluidine and
Tröger's-base-derived infinite co-ordination polymer microparticles.
You-Moon Jeon et al.
Small (Weinheim an der Bergstrasse, Germany), 5(1), 46-50 (2008-12-06)
Nicolas Claessens et al.
Journal of inorganic biochemistry, 101(7), 987-996 (2007-05-15)
Three stereoisomers of a Ru(II) complex bearing a chiral bis-phenanthroline Tröger's base analogue, TBphen2 (1), have been isolated from the reaction of the enantiomerically pure precursor complex Lambda- (or Delta-) cis-[Ru(phen)2(py)2]2+ (phen=1,10-phenanthroline, py=pyridine) with the racemic mixture of 1. Each
J Elguero et al.
Magnetic resonance in chemistry : MRC, 43(8), 665-669 (2005-06-30)
The (1)H and (13)C NMR spectra of two stereoisomeric bis-Tröger's bases and four stereoisomeric tris-Tröger's bases asymmetrically substituted on the external aromatic rings were recorded and the corresponding signals assigned. The relative configuration of the stereogenic units has been unequivocally
Carlos A M Abella et al.
The Journal of organic chemistry, 72(11), 4048-4054 (2007-05-03)
Using direct infusion electrospray ionization mass and tandem mass spectrometric experiments [ESI-MS(/MS)], we have performed on-line monitoring of some reactions used to form Tröger's bases. Key intermediates, either as cationic species or as protonated forms of neutral species, have been
Chromatograms
application for HPLCapplication for HPLCapplication for HPLCOur team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service