130346
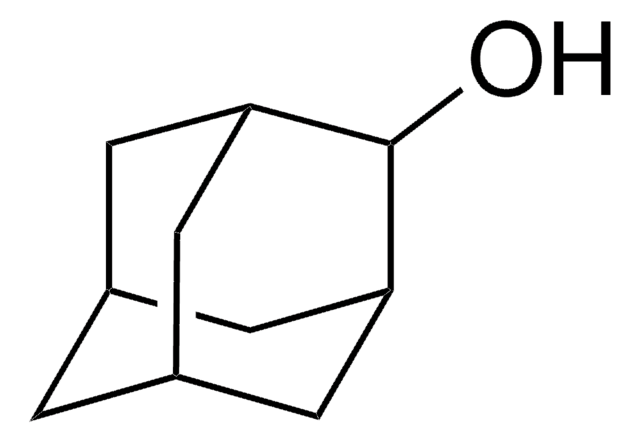
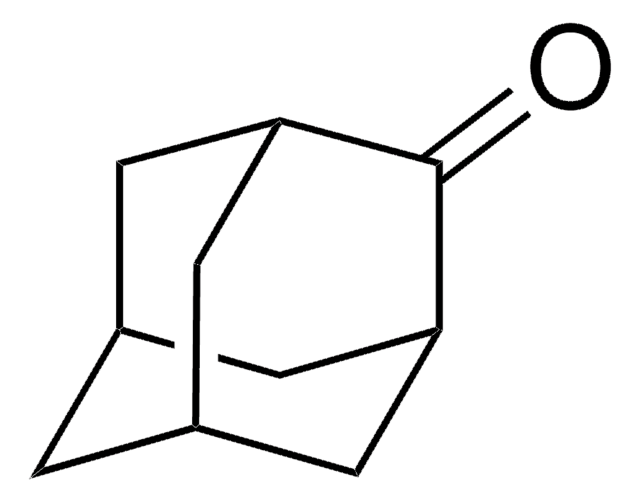
1-Adamantanol
ReagentPlus®, 99%
Synonym(s):
1-Hydroxyadamantane
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C10H16O
CAS Number:
Molecular Weight:
152.23
Beilstein:
1903952
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
product line
ReagentPlus®
Assay
99%
form
powder
mp
247 °C (subl.) (lit.)
SMILES string
OC12C[C@H]3C[C@H](C[C@H](C3)C1)C2
InChI
1S/C10H16O/c11-10-4-7-1-8(5-10)3-9(2-7)6-10/h7-9,11H,1-6H2/t7-,8+,9-,10-
InChI key
VLLNJDMHDJRNFK-CHIWXEEVSA-N
Looking for similar products? Visit Product Comparison Guide
Application
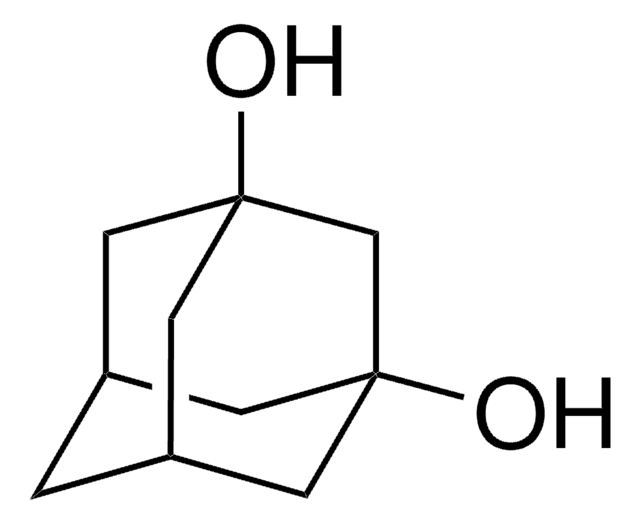
1-Adamantanol was used in the preparation of 1,3-adamantanediol.
Legal Information
ReagentPlus is a registered trademark of Merck KGaA, Darmstadt, Germany
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Koichi Mitsukura et al.
Journal of bioscience and bioengineering, 109(6), 550-553 (2010-05-18)
To efficiently produce 1,3-adamantanediol (1,3-ad(OH)(2)) from 1-adamantanol (1-adOH), our stocks of culture strains and soil microorganisms were surveyed for hydroxylation activity towards 1-adOH. Among them, the soil actinomycete SA8 showing the highest hydroxylation activity was identified as Streptomyces sp. based
Motowo Yamaguchi et al.
Inorganic chemistry, 45(20), 8342-8354 (2006-09-27)
New ruthenium(II) complexes having a tetradentate ligand such as tris(2-pyridylmethyl)amine (TPA), tris[2-(5-methoxycarbonyl)pyridylmethyl]amine [5-(MeOCO)3-TPA], tris(2-quinolylmethyl)amine (TQA), or bis(2-pyridylmethyl)glycinate (BPG) have been prepared. The reaction of the ligand with [RuCl2(Me2SO)4] resulted in a mixture of trans and cis isomers of the chloro(dimethyl
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service