All Photos(1)
About This Item
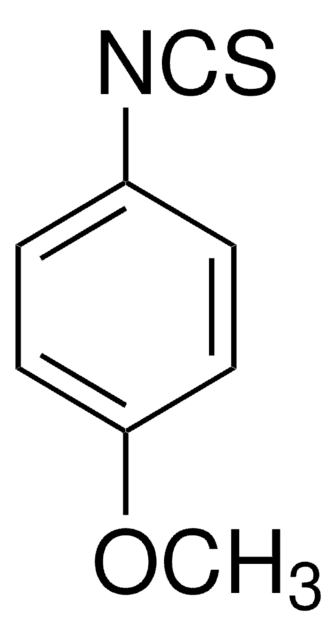
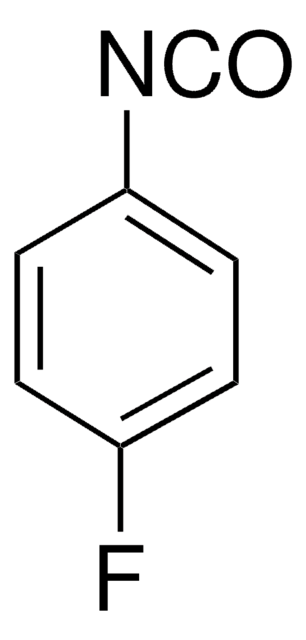
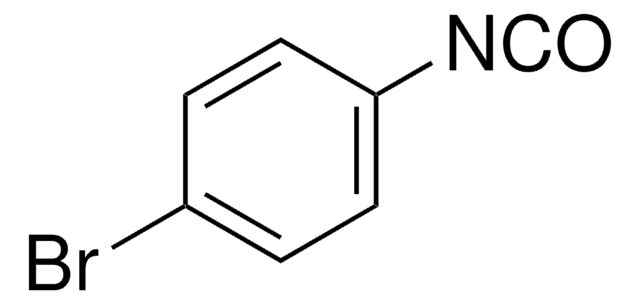
Linear Formula:
FC6H4NCS
CAS Number:
Molecular Weight:
153.18
Beilstein:
636596
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
form
solid
bp
228 °C (lit.)
mp
24-26 °C (lit.)
storage temp.
2-8°C
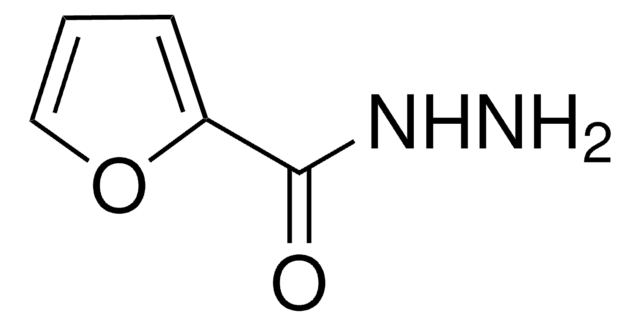
SMILES string
Fc1ccc(cc1)N=C=S
InChI
1S/C7H4FNS/c8-6-1-3-7(4-2-6)9-5-10/h1-4H
InChI key
NFIUJHJMCQQYDL-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
4-Fluorophenyl isothiocyanate has been used in the synthesis of thiourea derivatives. It has also been used in the preparation of -(4-phenylsulfonyl)-benzoic acid hydrazide.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Resp. Sens. 1 - Skin Corr. 1B - Skin Sens. 1
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
192.2 °F - closed cup
Flash Point(C)
89 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Meltem Yolal et al.
Bioorganicheskaia khimiia, 38(5), 610-620 (2013-01-25)
3-Fluoro-4-(4-phenylpiperazin-1-yl)aniline (II) prepared from 3,4-difluoro nitrobenzene was converted to the corresponding Schiff bases (III) and (IV) by treatment with 4-methoxybenzaldehyde and indol-3-carbaldehyde, respectively. Treatment of amine (II) with 4-fluorophenyl isothiocyanate affordedthe corresponding thiourea derivative (V). Compound (V) was converted to
María C Soraires Santacruz et al.
Bioorganic & medicinal chemistry, 25(15), 4055-4063 (2017-06-11)
A series of N
Fazila Rizvi et al.
Scientific reports, 9(1), 6738-6738 (2019-05-03)
A library of thiosemicarbazide derivatives of isoniazid 3-27, was synthesized and evaluated for their anti-inflammatory and urease inhibition activities, by using in vitro bioassays. Among these compounds 9, 10, 12, 21, and 26 were identified as new derivatives. Prolonged use
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service