All Photos(2)
About This Item
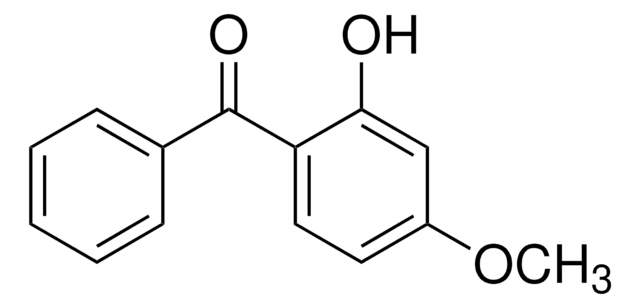
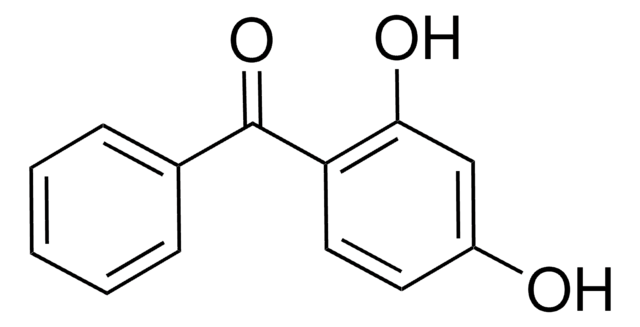
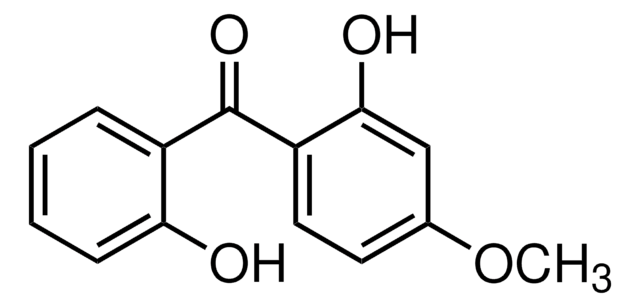
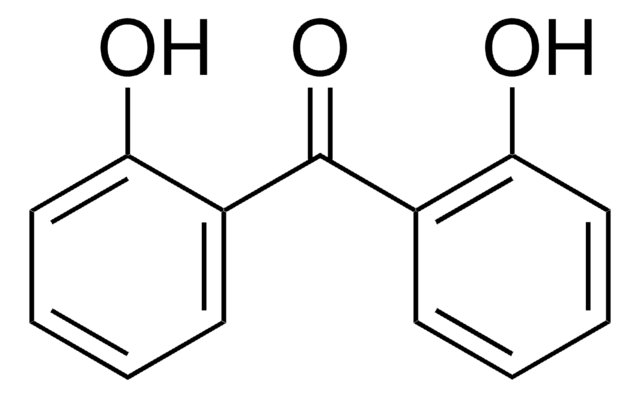
Linear Formula:
(HO)2C6H3COC6H5
CAS Number:
Molecular Weight:
214.22
Beilstein:
1311566
EC Number:
MDL number:
UNSPSC Code:
12162002
PubChem Substance ID:
NACRES:
NA.23
Recommended Products
Quality Level
Assay
99%
form
powder
mp
144.5-147 °C (lit.)
SMILES string
Oc1ccc(c(O)c1)C(=O)c2ccccc2
InChI
1S/C13H10O3/c14-10-6-7-11(12(15)8-10)13(16)9-4-2-1-3-5-9/h1-8,14-15H
InChI key
ZXDDPOHVAMWLBH-UHFFFAOYSA-N
Gene Information
rat ... Ar(24208)
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
- Substituent Effects on the Ultraviolet Absorption Properties of 2, 4-Dihydroxy Dibenzophenone: This research examines how different substituents affect the UV absorption characteristics of 2,4-dihydroxybenzophenone, a compound important for its UV protective properties (Wu et al., 2022).
- Kevlar® and Nomex modification via 2, 4-dihydroxybenzophenone anchoring improves water repellency and induces antibacterial and UV protection properties: Explores the enhancement of Kevlar and Nomex fabrics by anchoring 2,4-dihydroxybenzophenone to improve their functional properties (Tonis et al., 2023).
Legal Information
Kevlar is a registered trademark of E. I. du Pont de Nemours and Company
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Aquatic Chronic 2 - Eye Irrit. 2 - Repr. 2
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Flash Point(F)
257.0 °F
Flash Point(C)
125 °C
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Ting-Hao Kuo et al.
Analytica chimica acta, 1080, 95-103 (2019-08-15)
Structural analysis of biomolecules is essential to natural product discovery, especially for precious biomaterials such as agarwood. However, one of the greatest challenges to the characterization of natural products is the profound cost in time and manpower to the structural
Kresten Ole Kusk et al.
Environmental toxicology and chemistry, 30(4), 959-966 (2011-01-05)
Benzophenone (BP)-type ultraviolet (UV) filters are widely used in cosmetic and sunscreen products and can enter the aquatic environment. Therefore, we investigated the subchronic toxicity of 2,4-dihydroxybenzophenone (BP1) on the marine calanoid copepod Acartia tonsa in an early life-stage development
Min-Ah Park et al.
Toxicology, 305, 41-48 (2013-01-19)
2,4-Dihydroxybenzophenone (benzophenone-1; BP-1) is an UV stabilizer primarily used to prevent polymer degradation and deterioration in quality due to UV irradiation. Recently, BP-1 has been reported to bioaccumulate in human bodies by absorption through the skin and has the potential
L Fredriksen et al.
Applied and environmental microbiology, 85(6) (2019-01-13)
A two-domain GH10 xylanase-encoding gene (amor_gh10a) was discovered from a metagenomic data set, generated after in situ incubation of a lignocellulosic substrate in hot sediments on the sea floor of the Arctic Mid-Ocean Ridge (AMOR). AMOR_GH10A comprises a signal peptide
Natalie C Bamford et al.
Nature communications, 11(1), 2450-2450 (2020-05-18)
The exopolysaccharide galactosaminogalactan (GAG) is an important virulence factor of the fungal pathogen Aspergillus fumigatus. Deletion of a gene encoding a putative deacetylase, Agd3, leads to defects in GAG deacetylation, biofilm formation, and virulence. Here, we show that Agd3 deacetylates
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service