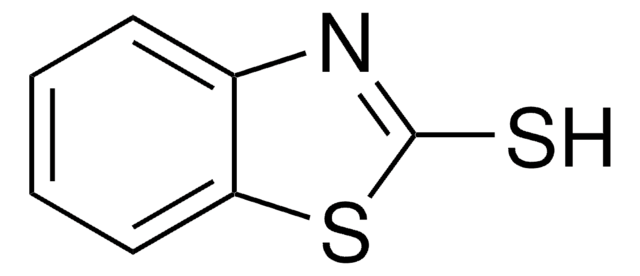
125571
5-Chloro-2-mercaptobenzothiazole
technical grade, ≥90%
Synonym(s):
5-Chloro-2-benzothiazolethiol, CMBT
About This Item
Recommended Products
grade
technical grade
Quality Level
Assay
≥90%
mp
198-200 °C (lit.)
SMILES string
Sc1nc2cc(Cl)ccc2s1
InChI
1S/C7H4ClNS2/c8-4-1-2-6-5(3-4)9-7(10)11-6/h1-3H,(H,9,10)
InChI key
NKYDKCVZNMNZCM-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Application
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Glycosylation is known to have profound influence on various physiochemical, cellular and biological functions of proteins. Alterations in this modification are known to affect the immune system and have been associated with various pathological states such as cancer, rheumatoid arthritis, and inflammatory diseases.
Mass Spectrometry of Glycans, method comparison and products
Protocols
The development of modern mass spectrometry (MS) equipment with high resolution and mass accuracy has led to its use in analyzing glycans for both profiling and structural studies.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service
![trans-2-[3-(4-tert-Butylphenyl)-2-methyl-2-propenylidene]malononitrile matrix substance for MALDI-MS, ≥99.0% (HPLC)](/deepweb/assets/sigmaaldrich/product/structures/249/587/f8021369-f65a-413d-887d-3c8a4d2a248f/640/f8021369-f65a-413d-887d-3c8a4d2a248f.png)