124974
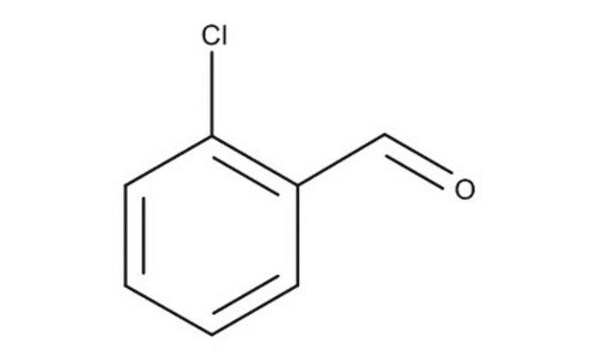
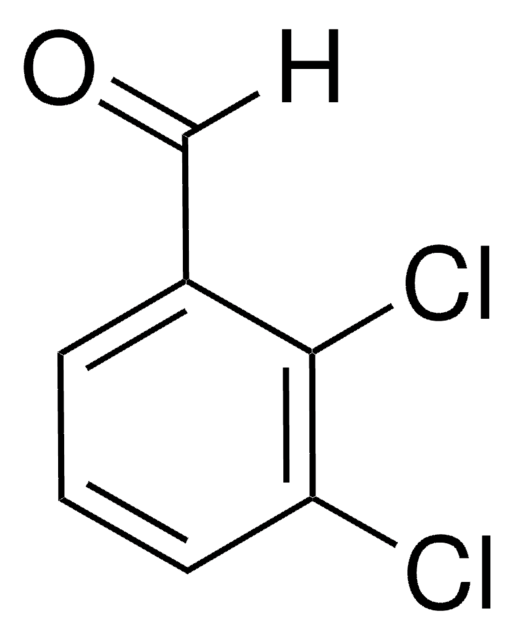
2-Chlorobenzaldehyde
99%
Synonym(s):
o-Chlorobenzaldehyde
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Linear Formula:
ClC6H4CHO
CAS Number:
Molecular Weight:
140.57
Beilstein:
385877
EC Number:
MDL number:
UNSPSC Code:
12352100
eCl@ss:
39050404
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
vapor density
4.84 (vs air)
Quality Level
vapor pressure
1.27 mmHg ( 50 °C)
Assay
99%
autoignition temp.
746 °F
refractive index
n20/D 1.566 (lit.)
bp
209-215 °C (lit.)
mp
9-11 °C (lit.)
density
1.248 g/mL at 25 °C (lit.)
SMILES string
[H]C(=O)c1ccccc1Cl
InChI
1S/C7H5ClO/c8-7-4-2-1-3-6(7)5-9/h1-5H
InChI key
FPYUJUBAXZAQNL-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
2-Chlorobenzaldehyde undergoes alkynylation with phenylacetylene in the presence of catalytic ligands and dimethylzinc at 0°C to form binaphthyl-derived amino alcohols.
Application
2-Chlorobenzaldehyde has been used in generation of small focused library of diversely functionalized dihydropyrimidine derivatives via one-pot three-component Biginelli cyclocondensation of β-ketoesters, aldehydes and thioureas.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Dam. 1 - Skin Corr. 1B - Skin Sens. 1
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 2
Flash Point(F)
206.6 °F - closed cup
Flash Point(C)
97 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Enantioselective alkynylation of aromatic aldehydes catalyzed by new chiral amino alcohol-based ligands.
Lu G, et al.
Tetrahedron Asymmetry, 12(15), 2147-2152 (2001)
Percutaneous absorption of 14C-labelled 2-chlorobenzaldehyde in rats. Metabolism and toxicokinetics.
E C Rietveld et al.
European journal of drug metabolism and pharmacokinetics, 13(4), 231-240 (1988-10-01)
2-Chlorobenzaldehyde might be produced when a moist skin is exposed to the riot control agent CS. CS-hydrolysis to 2-chlorobenzaldehyde and malononitrile occurs both in vitro and in vivo. No quantitative data have thus far been reported with respect to the
M Rogojerov et al.
Spectrochimica acta. Part A, Molecular and biomolecular spectroscopy, 61(7), 1661-1670 (2005-04-12)
Vibrational analysis of the two conformers of furfural and 2-chlorobenzaldehyde has been carried out on the basis of their IR and Raman spectra measured in isotropic and anisotropic (nematic liquid crystalline) solvent. The average orientation of the individual conformers in
M Anzaldi et al.
European journal of medicinal chemistry, 35(9), 797-803 (2000-09-28)
Vilsmeier reagents react with alpha/beta-ionones and carvone to produce aldehydes 7-11 in a one-step procedure. The indene derivative 11, which came from the double iminoalkylation of carvone and ring closure with the elimination of dimethylamine, was practically odourless, while all
Mathias J Jacobsen et al.
Organic letters, 13(13), 3418-3421 (2011-06-09)
A phosphine-mediated olefination of 2-alkynoates with aldehydes forming 1,3-dienes with high E-selectivity and up to 88% yield is described. Reaction conditions are optimized and reactions are demonstrated for various aryl, alkyl, and alkenyl aldehydes and for ethyl 2-alkynoates with different
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service