All Photos(1)
About This Item
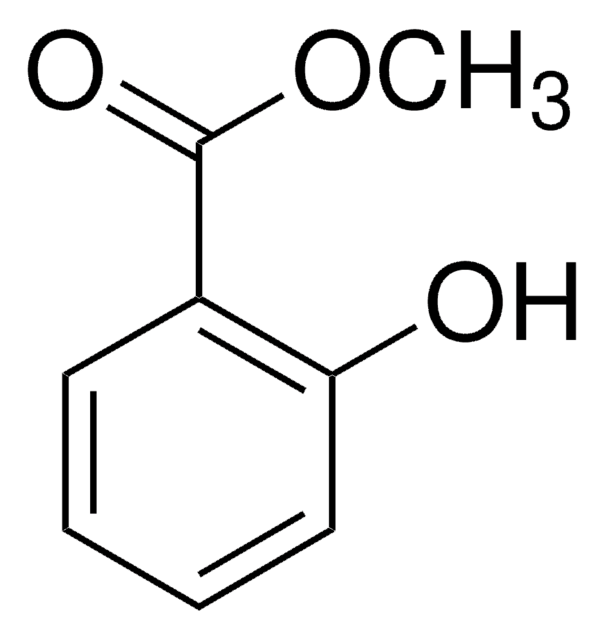
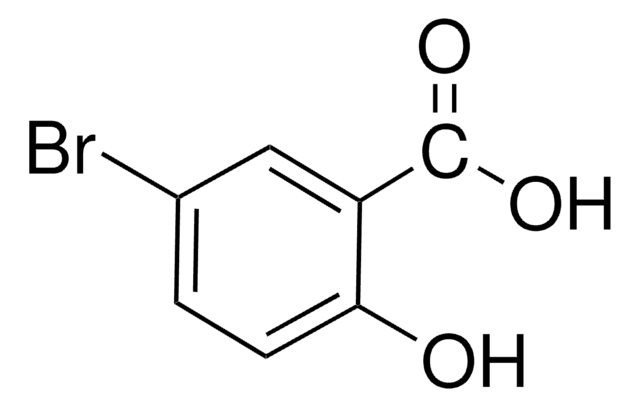
Linear Formula:
BrC6H3-2-(OH)CO2CH3
CAS Number:
Molecular Weight:
231.04
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
95%
form
solid
mp
61-65 °C (lit.)
functional group
bromo
SMILES string
COC(=O)c1cc(Br)ccc1O
InChI
1S/C8H7BrO3/c1-12-8(11)6-4-5(9)2-3-7(6)10/h2-4,10H,1H3
InChI key
FJYDBKPPGRZSOZ-UHFFFAOYSA-N
Related Categories
Application
Methyl 5-bromosalicylate was used as starting reagent in the synthesis of immobilization precursor of photoswitchable piperidine base.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Ragnar S Stoll et al.
Organic letters, 11(21), 4790-4793 (2009-10-02)
The synthesis of a photoswitchable piperidine base, carrying a monochlorosilane anchoring group, and its immobilization on silica gel, mimicking an oxide surface, is reported. Efficient photoswitching between the E and Z isomers of the azobenzene photochrome was demonstrated for the
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service