119792
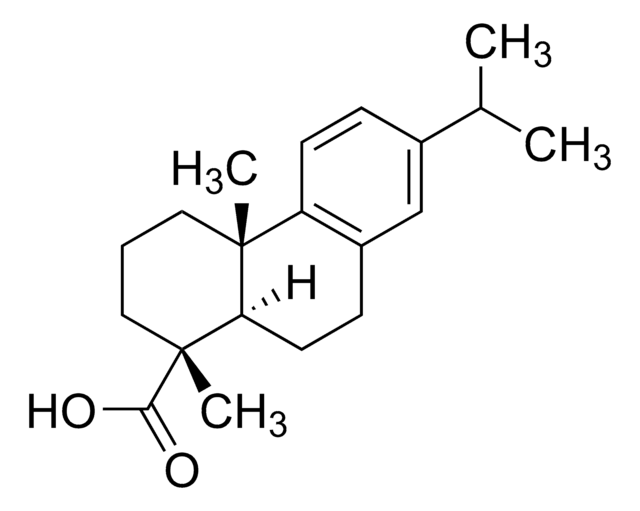
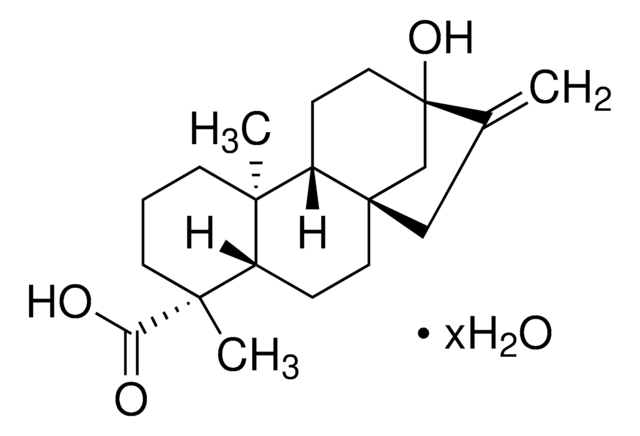
Podocarpic acid
98%
Synonym(s):
(+)-Podocarpic acid, (1S)-1,2,3,4,4a,9,10,10a-Octahydro-6-hydroxy-1,4a-dimethyl-1-phenanthrenecarboxylic acid, (1S,4aS,10aR)-1,2,3,4,4a,9,10,10a-Octahydro-6-hydroxy-1,4a-dimethyl-1-phenanthrenecarboxylic acid, Podocarpic acid (resin acid)
About This Item
Recommended Products
Quality Level
Assay
98%
form
solid
optical activity
[α]20/D +133°, c = 4 in ethanol
mp
193-196 °C (lit.)
SMILES string
[H][C@@]12CCc3ccc(O)cc3[C@@]1(C)CCC[C@]2(C)C(O)=O
InChI
1S/C17H22O3/c1-16-8-3-9-17(2,15(19)20)14(16)7-5-11-4-6-12(18)10-13(11)16/h4,6,10,14,18H,3,5,7-9H2,1-2H3,(H,19,20)/t14-,16-,17+/m1/s1
InChI key
VJILEYKNALCDDV-OIISXLGYSA-N
Gene Information
human ... TNF(7124)
Related Categories
Application
- (+)-Podocarpic acid as chiral template in the synthesis of aphidicolane, stemodane and stemarane diterpenoids: This article reviews the use of (+)-podocarpic acid in the synthesis of various diterpenoids, showcasing its utility in complex organic syntheses (La Bella et al., 2016).
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service