116238
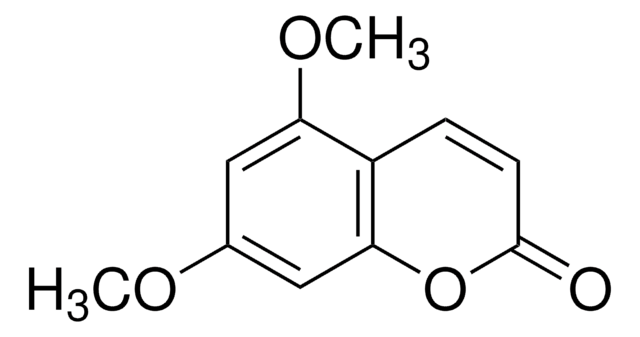
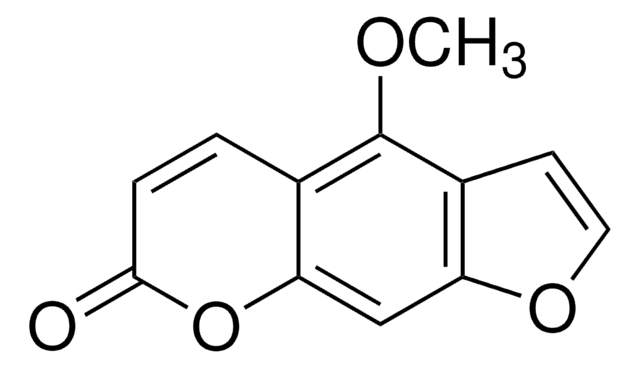
5,7-Dimethoxycoumarin
98%
Synonym(s):
Citropten, Limettin
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C11H10O4
CAS Number:
Molecular Weight:
206.19
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
form
solid
mp
146-149 °C (lit.)
SMILES string
COc1cc(OC)c2C=CC(=O)Oc2c1
InChI
1S/C11H10O4/c1-13-7-5-9(14-2)8-3-4-11(12)15-10(8)6-7/h3-6H,1-2H3
InChI key
NXJCRELRQHZBQA-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
5,7-dimethoxycoumarin is isolated and identified from leaves and fruits of Pelea anisata H. Mann, a plant whose fruit are used in the construction of mohikana leis. It induces frameshift mutagenesis in bacteria. It also causes lethal photosensitization and the formation of sister chromatid exchanges in Chinese hamster cells.
Biochem/physiol Actions
5,7-Dimethoxycoumarin induces the processes of differentiation and melanogenesis in murine (B16) and human (A375).
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Daniela Alesiani et al.
International journal of oncology, 32(2), 425-434 (2008-01-19)
In the present study we investigated the antiproliferative activity of 5,7-dimethoxycoumarin on the murine B16 and human A375 melanoma cell lines. The inhibitory concentration 50 (IC50) was estimated for each cell line by preliminary assay of tetrazolium salt reduction (MTT).
Earl Grey tea intoxication.
Josef Finsterer
Lancet (London, England), 359(9316), 1484-1484 (2002-05-04)
Ewa Chodurek et al.
Cellular & molecular biology letters, 17(4), 616-632 (2012-09-25)
Malignant melanoma (melanoma malignum) is one of the most dangerous types of tumor. It is very difficult to cure. In recent years, a lot of attention has been given to chemoprevention. This method uses natural and synthetic compounds to interfere
D Chouchi et al.
Journal of chromatography. A, 672(1-2), 177-183 (1994-06-24)
Generally on the gas chromatogram of a volatile essential oil, terpenes, oxygenated compounds and sesquiterpenes appear. With temperature programming, it was shown that some non-volatiles are present with the volatiles. They are simple coumarin (2H-1-benzopyran-2-one) derivatives such as citropten (5,7-dimethoxycoumarin)
Tomonori Nakamura et al.
Journal of natural medicines, 63(1), 15-20 (2008-07-09)
We have investigated the structure-activity relationship between 63 natural oxycoumarin derivatives and their effects on the expression of inducible-nitric oxide synthase (iNOS) induced by lipopolysaccharide. The protein expression of iNOS was screened by Western blot analysis, and four 5,7-dimethoxycoumarins were
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service