116157
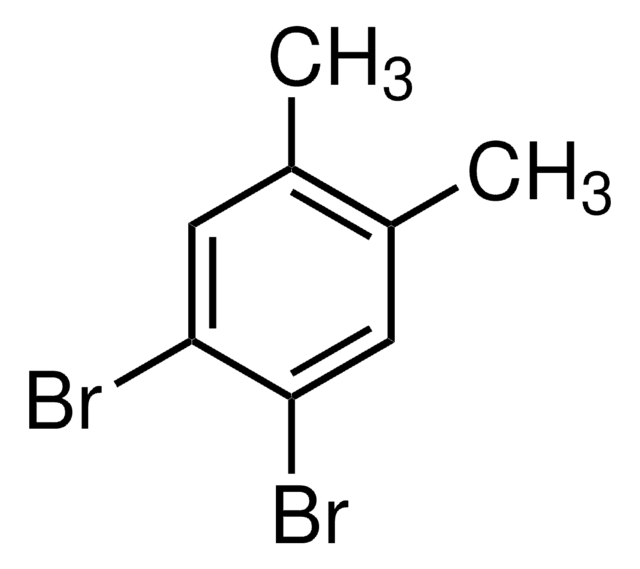
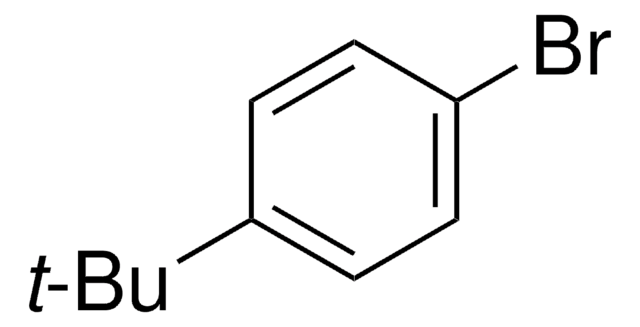
1,4-Dibromo-2,5-dimethylbenzene
98%
Synonym(s):
2,5-Dibromo-p-xylene
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
(CH3)2C6H2Br2
CAS Number:
Molecular Weight:
263.96
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
form
solid
bp
261 °C (lit.)
mp
72-74 °C (lit.)
SMILES string
Cc1cc(Br)c(C)cc1Br
InChI
1S/C8H8Br2/c1-5-3-8(10)6(2)4-7(5)9/h3-4H,1-2H3
InChI key
QENIALCDPFDFHX-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
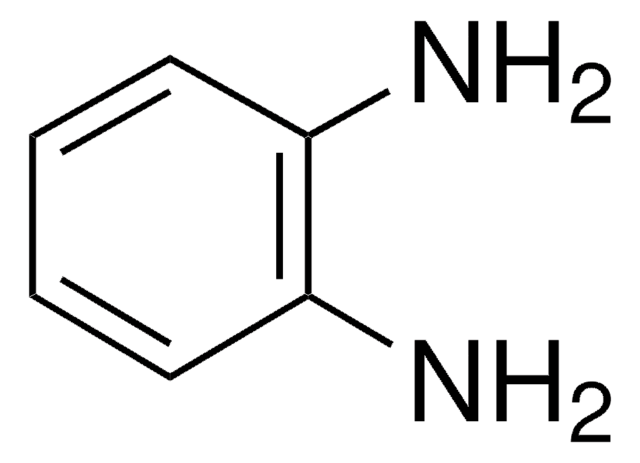
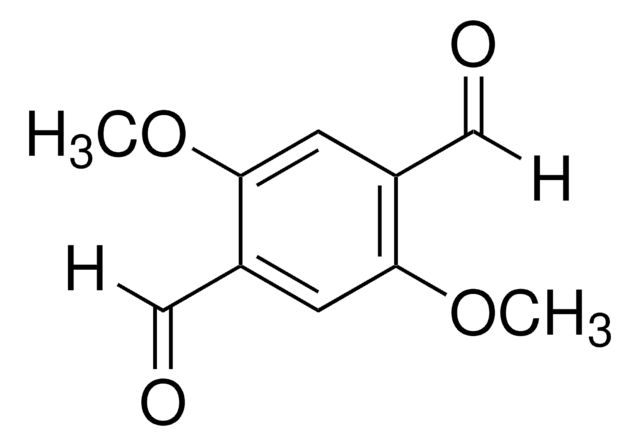
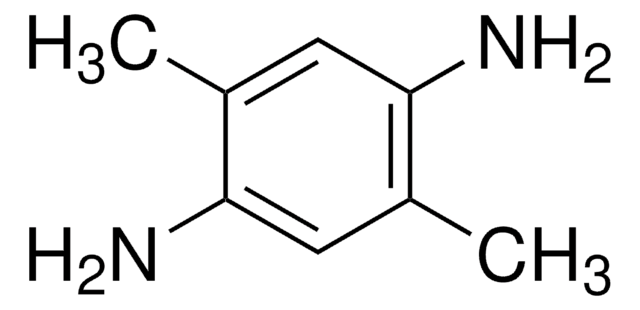
1,4-dibromo-2,5-dimethylbenzene has been used in the preparation of 4,4″-diformyl-2′,5′-dimethyl-1,1′.4′,1″-terphenyl. It has also been used in the preparation of 1,4-diformyl-2,5-dimethylbenzene.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Aquatic Chronic 2 - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Nikolai Kuhnert et al.
Organic & biomolecular chemistry, 1(7), 1157-1170 (2003-08-21)
The synthesis of aromatic dicarboxaldehydes, using dilithiation methodology is described along with their reactivity, in the [3 + 3] cyclocondensation reaction, with (1R,2R)-diaminocyclohexane to give trianglimine macrocycles. The scope and limitations of the cyclocondensation reaction are studied and some comments
Yuxiang Du et al.
Light, science & applications, 9, 151-151 (2020-09-10)
Tuneable microlasers that span the full visible spectrum, particularly red, green, and blue (RGB) colors, are of crucial importance for various optical devices. However, RGB microlasers usually operate in multimode because the mode selection strategy cannot be applied to the
Nikolai Kuhnert et al.
Organic & biomolecular chemistry, 3(10), 1911-1921 (2005-05-13)
The synthesis of aromatic dicarboxaldehydes is described along with their reactivity in the [3 + 3] cyclocondensation reaction with (1R,2R)-diaminocyclohexane to give trianglimine macrocycles. In particular, the scope and limitation of the reaction with regard to complete control of the
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service