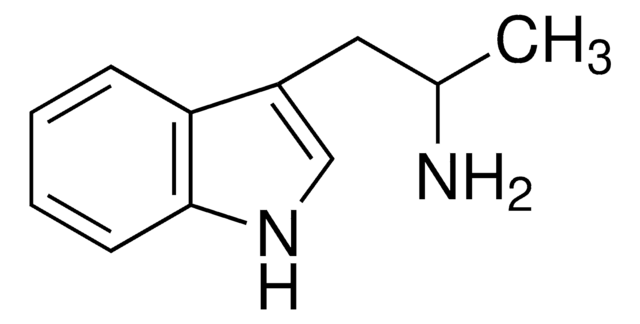
115312
N-ω-Methyltryptamine
99%
Synonym(s):
2-(Indol-3-yl)-N-methylethanamine, 3-(2-Methylaminoethyl)indole, 3-(2-[Methylamino]ethyl)indole, N-Monomethyltryptamine, Dipterine, N10-Methyltryptamine
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C11H14N2
CAS Number:
Molecular Weight:
174.24
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
99%
form
solid
mp
87-89 °C (lit.)
SMILES string
CNCCc1c[nH]c2ccccc12
InChI
1S/C11H14N2/c1-12-7-6-9-8-13-11-5-3-2-4-10(9)11/h2-5,8,12-13H,6-7H2,1H3
InChI key
NCIKQJBVUNUXLW-UHFFFAOYSA-N
Application
N-ω-Methyltryptamine was used in the preparation of N-acetyl-α−methyltryptamine.
N-ω-methyltryptamine was used in the biosynthesis of dolichantoside using U. tomentosa protein extracts.
Reactant for preparation of:
- Manzamine analogues for the control of neuroinflammation and cerebral infections
- Serotonin 4 receptors (5-HT4) receptor agonists
- A sulful-containing indole alkaloid, glypetelotine
- Selective inhibitors of cyclin dependent kinase (CDK4)
- Antagonist of the human tachykinin NK-2 receptor
- Inhibitors of the tyrosine-specific protein kinase pp60c-src SH2 Domain
Packaging
Bottomless glass bottle. Contents are inside inserted fused cone.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Strictosidine-related enzymes involved in the alkaloid biosynthesis of Uncaria tomentosa root cultures grown under oxidative stress.
Vera-Reyes I, Huerta-Heredia AA, Ponce-Noyola T, et al.
Biotechnology Progress, doi:10-doi:10 (2013)
Luigi Servillo et al.
Journal of agricultural and food chemistry, 60(37), 9512-9518 (2012-09-11)
The occurrence of N-methylated tryptamine derivatives in bergamot plant (Citrus bergamia Risso et Poit) is reported for the first time. Interestingly, the most abundant of these substances is N,N,N-trimethyltryptamine, which has not been previously identified in any citrus plant. The
K J Willis et al.
Biophysical journal, 57(2), 183-189 (1990-02-01)
Direct and indirect methods are described to combine steady-state and picosecond time-resolved fluorescence decay data to generate decay-associated excitation spectra. The heterogeneous fluorescence from a fluorophore mixture that models protein fluorescence was resolved into individual component excitation spectra. The two
T J Williams et al.
European journal of pharmacology, 245(3), 197-201 (1993-05-15)
The binding of [3H]5-hydroxytryptamine (5-HT) to rat enteric membranes was inhibited by the inclusion of 5-HT 2-methyl-5-HT, 5-hydroxytryptophan, N,N,N-triethyltryptamine and 2-Br-N,N-diethyltryptamine in the incubation buffer. In contrast, tryptamine, 5-methoxytryptamine and 2-methyl-N,N-diethyltryptamine enhanced binding. Ascorbate and dithiothreitol facilitated and reduced binding
M C Oon et al.
Psychopharmacology, 54(2), 171-175 (1977-10-20)
The hallucinogenic substance N',N'-dimethyltryptamine and its precursor N-methyltryptamine were found in 24-h specimens of urine from 19 normal human subjects; the mean excretion rates were 386 ng 24 h(-1) and 856 ng 24 h(-1) respectively. The urinary excretion of both
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service

![1,2,3,4-Tetrahydro-9H-pyrido[3,4-b]indole 98%](/deepweb/assets/sigmaaldrich/product/structures/181/460/3d58bc34-1b5c-4295-bbac-3b52085670e8/640/3d58bc34-1b5c-4295-bbac-3b52085670e8.png)