112186
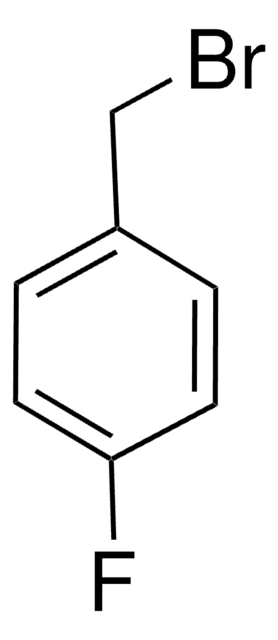
4-Bromobenzyl bromide
98%
Synonym(s):
α,4-Dibromotoluene
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
BrC6H4CH2Br
CAS Number:
Molecular Weight:
249.93
Beilstein:
606498
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
form
solid
mp
62-64 °C (lit.)
SMILES string
BrCc1ccc(Br)cc1
InChI
1S/C7H6Br2/c8-5-6-1-3-7(9)4-2-6/h1-4H,5H2
InChI key
YLRBJYMANQKEAW-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
4-Bromobenzyl bromide was used to prepare a 20-member aminomethyl-substituted biaryl library via sequential N-alkylation of various amines.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Resp. Sens. 1 - Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
M G Organ et al.
Journal of combinatorial chemistry, 3(5), 473-476 (2001-09-11)
Described herein is the semiautomated preparation of a 20-member aminomethyl-substituted biaryl library. The two-step solution-phase synthesis was achieved via sequential N-alkylation of various amines with either 3- or 4-bromobenzyl bromide and Suzuki cross-coupling of the resultant aryl bromides with various
E V Sargent et al.
Drug and chemical toxicology, 22(4), 583-593 (1999-10-28)
As part of an occupational hazard evaluation, p-bromobenzyl bromide (p-BBB) was evaluated for genotoxic activity in the Ames microbial mutagenicity assay, the alkaline elution assay for DNA strand breaks in rat hepatocytes and the in vitro chromosome aberration assay in
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service