All Photos(2)
About This Item
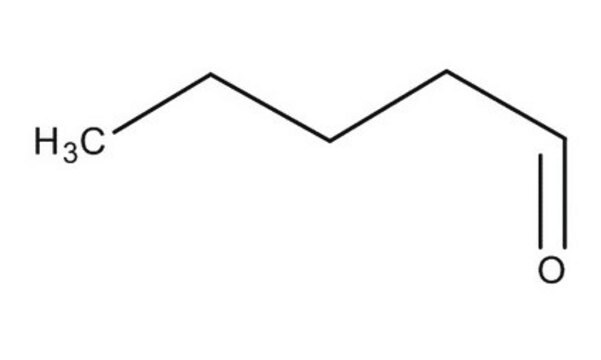
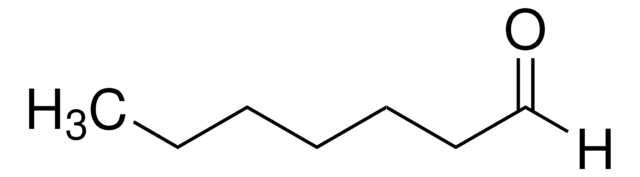
Linear Formula:
CH3(CH2)3CHO
CAS Number:
Molecular Weight:
86.13
Beilstein:
1616304
EC Number:
MDL number:
UNSPSC Code:
12352100
eCl@ss:
39021107
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
autoignition temp.
428 °F
refractive index
n20/D 1.394 (lit.)
bp
102-103 °C (lit.)
mp
−92 °C (lit.)
density
0.81 g/mL at 25 °C (lit.)
SMILES string
[H]C(=O)CCCC
InChI
1S/C5H10O/c1-2-3-4-5-6/h5H,2-4H2,1H3
InChI key
HGBOYTHUEUWSSQ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Valeraldehyde also known as pentanal, serves as a precursor or intermediate to build more complex organic molecules.
Application
Valeraldehyde has been used to study its time-weighted average sampling using solid-phase microextraction (SPME) device.
Valeraldehyde is used as a gaseous standard in the study of the sorptive loss pattern for volatile compounds.
Valeraldehyde is used in flavoring compounds and as a rubber accelerator.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Inhalation - Eye Irrit. 2 - Flam. Liq. 2 - Skin Sens. 1 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
3 - Flammable liquids
WGK
WGK 1
Flash Point(F)
44.6 °F
Flash Point(C)
7 °C
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Yong-Hyun Kim et al.
Journal of separation science, 35(21), 2914-2921 (2012-10-16)
In this study, the sorptive loss patterns for volatile organic compounds were evaluated by gaseous standards containing 13 compounds (benzene, toluene, styrene, p-xylene, methyl ethyl ketone, methyl isobutyl ketone, isobutyl alcohol, butyl acetate, acetaldehyde, propionaldehyde, butyraldehyde, isovaleraldehyde, and valeraldehyde). The
J B Webster et al.
Journal of food science, 74(9), S390-S398 (2010-05-25)
Milk packaged in glass bottles overwrapped with iridescent films (treatments blocked either a single visible riboflavin [Rb] excitation wavelength or all visible Rb excitation wavelengths; all treatments blocked UV Rb excitation wavelengths) was exposed to fluorescent lighting at 4 degrees
S W Tsai et al.
Journal of chromatography. A, 954(1-2), 191-198 (2002-06-13)
A solid-phase microextraction (SPME) device was used as a time-weighted average sampler for n-valeraldehyde. The SPME device was first modified to improve the wearer's acceptance as a passive sampler. Then a poly(dimethylsiloxane)-divinylbenzene fiber was used and O-2,3,4,5,6-(pentafluorobenzyl)hydroxylamine hydrochloride (PFBHA) was
Masayuki Fujita et al.
Plant & cell physiology, 44(5), 481-490 (2003-05-30)
Induction of pumpkin (Cucurbita maxima Duch.) glutathione S-transferase (GST, EC 2.5.1.18) by aldehydes and related compounds was examined. All of the tested compounds induced pumpkin GST to different degrees, and it was found that (1) aldehydes induce GST directly and
P Scalabrini et al.
International journal of food microbiology, 39(3), 213-219 (1998-04-29)
Soybean milk, which serves as a base for a variety of beverages, contains raffinose, stachyose, pentanal and n-hexanal; the former two may be responsible for flatulence after fermentation, whilst the latter two for a beany flavour. Twenty-seven strains of Bifidobacterium
Protocols
-Tolualdehyde; Valeraldehyde; Isovaleraldehyde
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service