All Photos(1)
About This Item
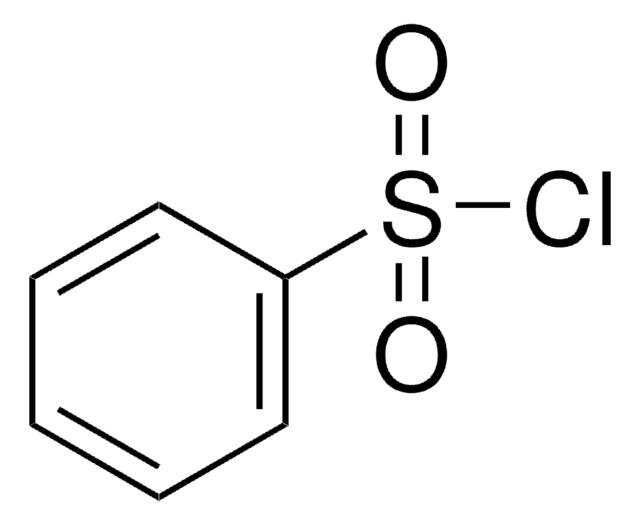
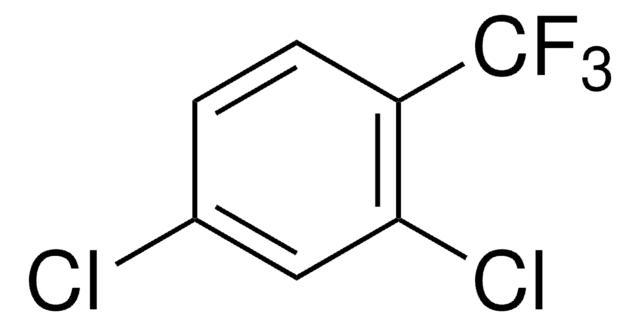
Linear Formula:
C6H5SO2Cl
CAS Number:
Molecular Weight:
176.62
Beilstein:
606926
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
vapor pressure
0.04 mmHg ( 20 °C)
Quality Level
Assay
96%
refractive index
n20/D 1.551 (lit.)
bp
251-252 °C (lit.)
mp
13-15 °C (lit.)
solubility
alcohol: soluble
cold water: insoluble
diethyl ether: soluble
density
1.384 g/mL at 25 °C (lit.)
SMILES string
ClS(=O)(=O)c1ccccc1
InChI
1S/C6H5ClO2S/c7-10(8,9)6-4-2-1-3-5-6/h1-5H
InChI key
CSKNSYBAZOQPLR-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Benzenesulfonyl chloride reacts with Grignard reagent from N-unsubstituted indoles to form oxindoles or substituted indoles. It is the derivatization reagent for the determination of various amines in waste water and surface water at the sub-ppb level by gas chromatography-mass spectrometry.
Application
Benzenesulfonyl chloride may be used in thiamine assay for determination of thiamine in different food products.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Dam. 1 - Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 1
Flash Point(F)
260.6 °F
Flash Point(C)
127 °C
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Analysis of primary and secondary aliphatic amines in waste water and surface water by gas chromatography-mass spectrometry after derivatization with 2, 4-dinitrofluorobenzene or benzenesulfonyl chloride.
Sacher F, et al.
Journal of Chromatography A, 764(1), 85-93 (1997)
Unusual reactions of magnesium indolates with benzenesulfonyl chloride.
Wenkert E, et al.
The Journal of Organic Chemistry, 52(15), 3404-3409 (1987)
H Kataoka et al.
Biomedical chromatography : BMC, 6(5), 251-254 (1992-09-01)
A selective and sensitive method for the determination of low molecular weight aliphatic primary amines in urine is described. These amines were converted into their benzenesulphonyl derivatives by a modified Hinsberg procedure, and measured by gas chromatography with flame photometric
Mario O Salazar et al.
Molecular diversity, 15(3), 713-719 (2011-01-06)
A semisynthetic β-glucosidase inhibitor was identified from a chemically engineered extract prepared by reaction with benzenesulfonyl chloride. The structure includes a natural histamine portion and a benzenesulfonyl portion introduced during the diversification step.
A G Soliman
Journal - Association of Official Analytical Chemists, 64(3), 616-622 (1981-05-01)
A semiautomated procedure was used to measure the fluorescence of sample extracts before and after the addition of benzenesulfonyl chloride (BSC). Addition of BSC inhibited thiochrome formation and provided a more representative blank based on the fluorescence of all the
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service