103276
Harmane
98%
Synonym(s):
1-Methyl-9H-pyrido[3,4-b]indole, 2-Methyl-β-carboline, Aribine
About This Item
Recommended Products
Quality Level
Assay
98%
drug control
stupéfiant (France)
mp
235-238 °C (lit.)
solubility
methanol: soluble 50mg/ml
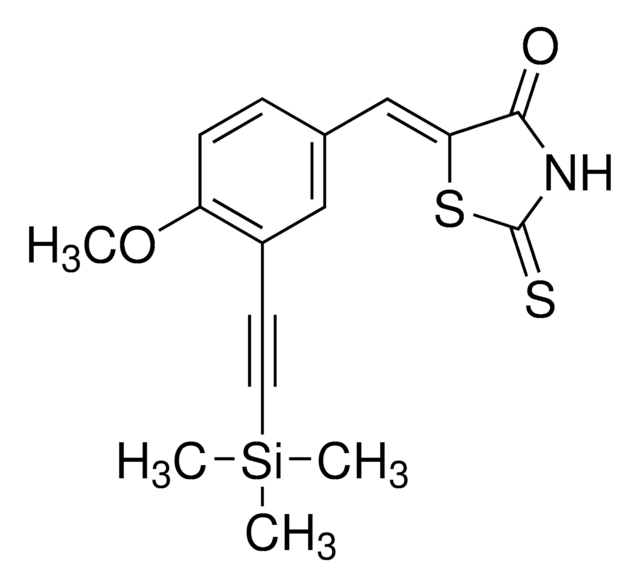
SMILES string
Cc1nccc2c3ccccc3[nH]c12
InChI
1S/C12H10N2/c1-8-12-10(6-7-13-8)9-4-2-3-5-11(9)14-12/h2-7,14H,1H3
InChI key
PSFDQSOCUJVVGF-UHFFFAOYSA-N
Gene Information
human ... CYP2D6(1565)
rat ... Gabra2(29706)
Looking for similar products? Visit Product Comparison Guide
General description
- Harmane is a potent tremor-producing β-carboline alkaloid and neurotoxin.
- It is major representative of heterocyclic aromatic amines, a group of mutagenic and carcinogenic substances which are formed in meat from the precursors creatine, creatinine, amino acids and sugars during the heating at high temperatures.
- Blood harmane concentration is elevated in essential tremor, late-life neurological disease.
Application
- Harmane was used in trace level determination of harmane by planar chromatography coupled with (tandem) mass spectrometry.
- It was used to study interactions of norharman and harman with DNA.
- It may be used as matrix for analysis of cyclodextrins and for sulfated oligosaccharides in combination with DHB as co-matrix.
Biochem/physiol Actions
Preparation Note
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Skin Irrit. 2
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Glycosylation is known to have profound influence on various physiochemical, cellular and biological functions of proteins. Alterations in this modification are known to affect the immune system and have been associated with various pathological states such as cancer, rheumatoid arthritis, and inflammatory diseases.
Mass Spectrometry of Glycans, method comparison and products
Protocols
The development of modern mass spectrometry (MS) equipment with high resolution and mass accuracy has led to its use in analyzing glycans for both profiling and structural studies.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service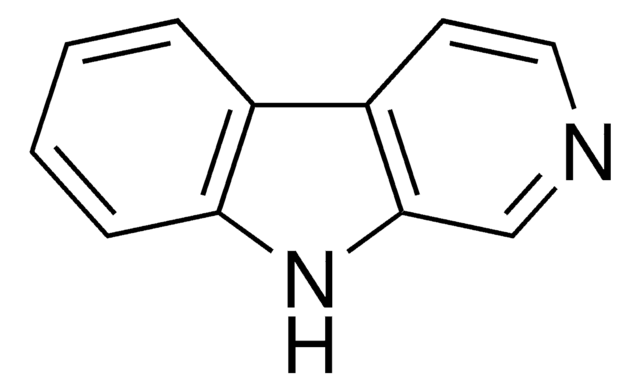
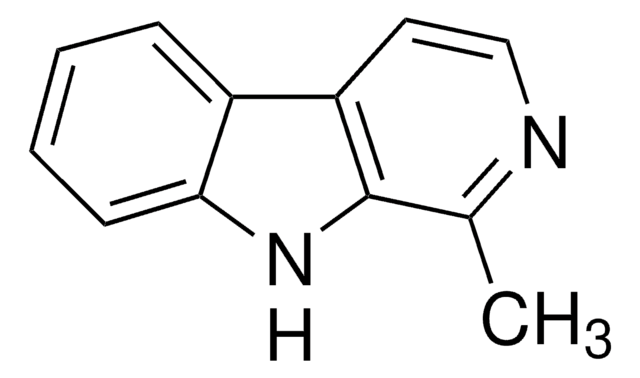
![1,2,3,4-Tetrahydro-9H-pyrido[3,4-b]indole 98%](/deepweb/assets/sigmaaldrich/product/structures/181/460/3d58bc34-1b5c-4295-bbac-3b52085670e8/640/3d58bc34-1b5c-4295-bbac-3b52085670e8.png)
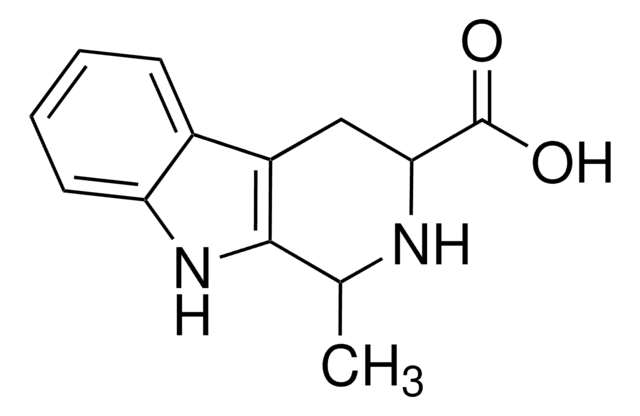
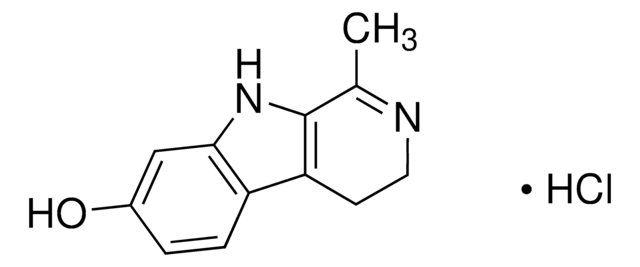
![9H-pyrido[2,3-b]indole AldrichCPR](/deepweb/assets/sigmaaldrich/product/structures/418/992/8c7bac06-11e8-45d7-b863-5d35b582e871/640/8c7bac06-11e8-45d7-b863-5d35b582e871.png)

![2,3,4,5-Tetrahydro-1H-pyrido[4,3-b]indole AldrichCPR](/deepweb/assets/sigmaaldrich/product/structures/376/664/07577eb6-6e8c-4237-b8c5-03da4c8e7d88/640/07577eb6-6e8c-4237-b8c5-03da4c8e7d88.png)